Retina
Real-World Faricimab nAMD Outcomes
First-year results promising for efficacy and extending treatment intervals.

Howard Larkin
Published: Tuesday, October 1, 2024
Patients with neovascular age-related macular degeneration (nAMD) treated with faricimab on average saw visual acuity improve or stabilise, and retina central subfield thickness (CST) decrease during their first year of treatment, a large retrospective study indicates. Many also were able to extend the interval between faricimab injections while apparently maintaining disease control.
Faricimab is the first bispecific monoclonal antibody for treating nAMD and diabetic macular oedema. In addition to targeting vascular endothelial growth factor (VEGF), it blocks angiopoietin-2, stabilising blood vessels and reducing leakage and inflammation, which theoretically could increase faricimab’s efficacy compared with anti-VEGF-only alternatives. It is also designed to reduce the risk of systemic exposure and inflammation.
Large cohort
A real-world retrospective study reported by David Tabano MD examined electronic medical records of more than 19,000 eyes of nearly 16,000 nAMD patients treated with faricimab injections for at least 12 months. It included nearly 15,000 patients previously treated for nAMD with other anti-VEGF agents and about 1,100 who were treatment naïve. The records were drawn from the American Academy of Ophthalmology’s Intelligent Research in Sight (IRIS) registry, which includes data on more than 75 million unique ophthalmic patients seen by 16,000 clinicians across the US.
All patients had at least 12 months of medical record data prior to receiving faricimab, including at least two measurements of visual acuity and two CST measurements for a CST subgroup. About 42% of treatment-naïve eyes and 52% of previously treated eyes had best documented visual acuity (BDVA) of 20/40 or better at the time of first treatment with faricimab. Previously treated eyes received about seven injections at about 45-day intervals in the 12 months prior to switching to faricimab. Two-thirds of eyes previously used aflibercept.
Positive outcomes
Overall, the study found that mean BDVA improved slightly for treatment-naïve patients, rising an average of nearly 3 letters after five faricimab injections; while previously treated patients were stable, losing 0.3 lines after five treatments. These findings are consistent with other studies that have seen an improvement in visual acuity early in treatment, followed by preservation later in treatment, Dr Tabano said.
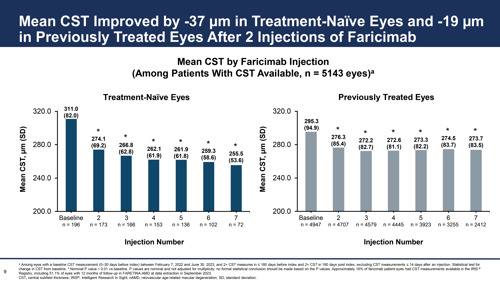
Similarly, CST improved more in treatment-naïve eyes, steadily declining from a mean 311.0 μ at treatment outset to 262.1 μ after four injections. By comparison, previously treated eyes declined from a mean CST of 295.3 μ to 272.6 μ after four treatments, which was in addition to any reduction they had seen on the previous treatment, Dr Tabano pointed out. Nearly two-thirds of treatment-naïve eyes and more than one-third of previously treated eyes saw CST reduced by 10% or more following faricimab initiation.
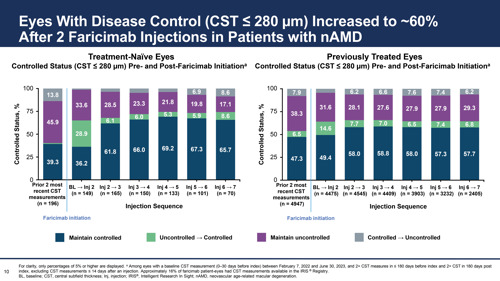
Furthermore, the percentage of eyes not controlled dropped for both groups after faricimab was initiated, from 46% to 17% after four injections in treatment-naïve eyes and 38% to 29% in previously treated eyes.
Fewer injections were needed to control nAMD after the first 6 months of treatment with faricimab, falling from a mean of 4.0 to 2.4 in the second 6 months for treatment-naïve eyes, and from 4.3 to 3.2 in previously treated eyes. In particular, treatment-naïve eyes saw comparable injection frequency in 12 months to the Tenaya and Lucerne phase III clinical trials for faricimab. This suggests physicians may be comfortable extending treatment intervals based on early anatomical response, although Dr Tabano said this is not certain because the rationale for extending is not included in the electronic records.
The results suggest real-world effectiveness and durability of faricimab treatment, which could effectively increase system capacity to treat nAMD, Dr Tabano concluded.
Dr Tabano presented at ARVO 2024 in Seattle, US.
David Tabano PhD is principal health economist at Genentech, South San Francisco, US. tabano.david@gene.com