CATARACT SURGERY IN EYES WITH DISEASED CORNEAS
Damaged corneas can interrupt what should be an uneventful surgery

Soosan Jacob
Published: Wednesday, February 26, 2020
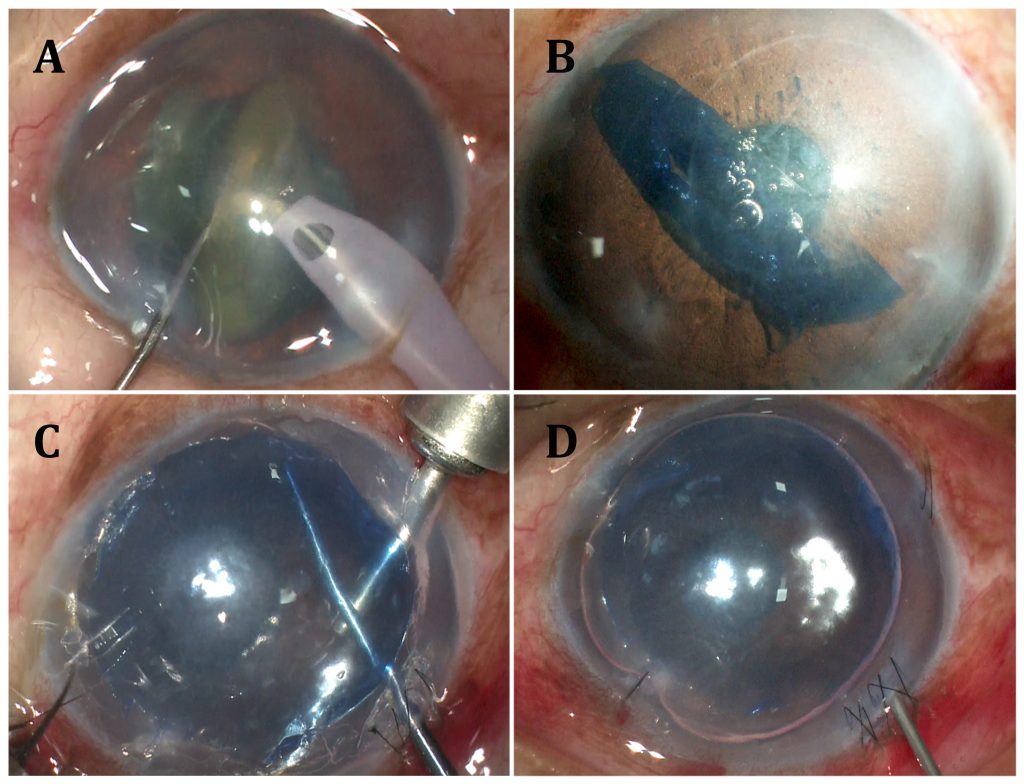 Fig: A- Nucleus emulsification, cortex aspiration and in-the-bag IOL placement performed in an eye with Fuchs’ Endothelial Corneal dystrophy; B- Pre-Descemet’s Endothelial Keratoplasty (PDEK) graft injected into anterior chamber and graft orientation determined using endoilluminator assisted PDEK technique; C- Graft centred and edge folds removed using air pump assisted PDEK technique. Host Descemetic scaffolding seen at inferior 6o’clock position; D- Well placed PDEK graft seen with 80% air fill.
The cornea is the window to the world. It’s a beautiful structure that is also the cataract surgeon’s window to the patient’s eye. It gives a crystal clear, unimpeded, magnified view of the internal structures while acting as the roof to a stable chamber, allowing uneventful surgery. However, we do sometimes come across cataracts in eyes that have some defect in the cornea. In this article, I take a look at some of the problems faced and how
we can manage them.
Fuchs’ Endothelial
Corneal Disease (FECD)
One common situation we sometimes face is the development of cataract in patients with FECD. The questions here of course are – first, whether cataract surgery could worsen the already compromised endothelium and second, if it is better to remove the cataract early when it is soft and requires less phaco energy. If visual loss is not present and the patient is asymptomatic, I generally prefer to advise only observation, sometimes also adding medical management in the form of Rho kinase inhibitors for endothelial regeneration, hypertonic saline to deturgesce the cornea and anti-glaucoma medications to decrease endothelial stress.
If vision starts to decrease or the patient becomes symptomatic, I like to proceed based on grade of FECD, cataract density and estimated contribution to visual loss from each. With minimal endothelial changes and a soft cataract, phacoemulsification can usually be done uneventfully using the least possible phaco energy. I prefer torsional ultrasound and use Arshinoff’s soft shell technique using dispersive and cohesive viscoelastic. I try to debulk the nucleus within the bag before bringing fragments out for iris plane emulsification.
Soft cataracts can be removed easily using high vacuum with very low energy. Dispersive OVD should be intermittently replenished for endothelial coating and wound burns should be avoided. I prefer an extracapsular cataract extraction or manual small-incision cataract surgery under dispersive viscoelastic cover for dense cataracts that would require excessive phaco energy.
Hydrophobic IOL is preferred as hydrophilic IOLs can opacify if air/gas tamponade is required during a possible future endothelial keratoplasty (EK). Preoperative counselling regarding possible slower postoperative recovery as well as possible need for an EK is important even with soft cataracts.
With decompensated FECD, I combine phacoemulsification with in-the-bag IOL placement and an EK irrespective of the grade of cataract.
Though Descemet’s stripping automated EK (DSAEK) and Descemet’s Membrane EK (DMEK) may be performed, my personal preference is pre-Descemet’s EK (PDEK), utilising three techniques that I have described to increase success and repeatability – endoilluminator-assisted PDEK, air pump-assisted PDEK and host Descemetic scaffolding.
I prefer to remove a non-cataractous crystalline lens too as the risk of cataract developing secondary to air tamponade, prolonged steroid usage, inflammation, natural ageing etc. is high and can result in the need for surgery, which can in turn cause loss of precious graft endothelial cells.
Cataract surgery can, however, be challenging in FECD due to poor visibility. Removing the oedematous epithelium can help. Increased stromal oedema whenever the irrigation probe enters the anterior chamber together with any pre-existing haze or minimal stromal scarring decreases visibility in advanced cases. A good quality microscope, capsular staining with trypan blue and use of an endoilluminator as an external oblique light source can help. For a successful EK, it is better to emulsify the cataract working closer to the endothelium than to the posterior capsule to avoid a posterior capsular rent.
Patients with advanced cornea guttata, irido-corneal-endothelial (ICE) syndrome, severe toxic anterior segment syndrome (TASS) and other causes of endothelial dysfunction such as prolonged inflammation or trauma may also be handled using above described principles. Some of these conditions may have peripheral anterior synechiae and glaucoma which may need to be addressed simultaneously. The air pump-assisted PDEK technique allows synechiolysis under air tamponade and prevents bleeding from the iris, hyphema and a fibrinous atmosphere. It helps achieve effective intraoperative graft attachment and therefore gives a greater chance of success even when combined glaucoma surgery is planned.
Scarred cornea
Patients with cataract and stromal and endothelial scarring may require cataract extraction combined with penetrating keratoplasty. This allows better intraoperative visibility, prompt visual rehabilitation after surgery and avoids later cataract extraction and graft endothelial loss. Major disadvantages are inability to accurately estimate IOL power because of unpredictable postoperative keratometry and suture-induced postoperative astigmatism.
Though not very accurate, with experience, it is possible to keep the predicted mean postoperative keratometry within an acceptable range to avoid very large refractive errors. I have found the Maloney intraoperative keratometer very useful to keep suture-related astigmatism to acceptable levels. I also utilise postoperative suture adjustment in the form of selective suture removal or suture replacement for astigmatism that decreases visual acuity.
Cataract extraction may be done open-sky when view is poor. If view permits, closed-chamber phacoemulsification followed by a penetrating keratoplasty can decrease open sky time and allow a rhexis and in-the-bag IOL placement. Phaco incisions should be scleral or limbal and short. Postoperative refractive correction in the form of spectacles or rigid contact lenses may be given.
Postponing cataract extraction to after the keratoplasty allows correction of large refractive errors, but results in endothelial loss at the time of cataract surgery and also requires waiting until complete suture removal resulting in delayed visual rehabilitation.
Corneal dystrophies
and superficial scarring
In case of stromal scarring (not involving Descemet’s-endothelial complex) contributing to a significant decrease in vision, phototherapeutic keratectomy or superficial/deep anterior lamellar keratoplasty (ALK) may be indicated for treatment. I perform this first and defer cataract surgery until after complete suture removal and stabilisation of the refractive error, since endothelial loss is not a major factor.
If, however, the cataract is very dense, depending on extent, level and density of opacity, combined surgery may be done either using techniques that enhance visualisation through scarred corneas or by dissecting the anterior stromal scar by an ALK to a level that allows better visualisation, performing cataract extraction and then proceeding for deeper stromal dissection. If the scar is off the visual axis, or in case of very faint scarring, I perform only cataract surgery.
Irregular cornea
Another situation that occasionally presents is cataract in irregular corneas. This could be secondary to a variety of conditions such as keratoconus, pellucid marginal degeneration, post-LASIK ectasia, radial keratotomy, post-trauma, post-surgery such as after LASIK, keratoplasty, Intacs, cross-linking etc.
In these corneas, when the cataract is soft, I like to do a rigid gas permeable contact lens trial and retinal acuity meter to estimate potential best-corrected visual acuity. Corneal topography is important and multiple measurements should be taken to ensure repeatability. Corneal HOA analysis and pupillometry are also important.
The major problem here is IOL power calculation. In the case of LASIK, IOL power is underestimated post-myopic LASIK and overestimated post-hyperopic LASIK. Post-myopic LASIK, total corneal power measured by ray tracing can be used for better IOL power prediction. Wang-Koch-Hill ASCRS online calculator; Barrett True K No History; No History Shammas-PL; Haigis-L and other methods are useful. Aramberri Double K method corrects effective lens position in SRK/T, Holladay-1 and Hoffer-Q formulas. Hoffer-Savini LASIK IOL power tool allows simultaneous calculation of various formulas to obtain possible K values. Agreement between various K values should be checked while erring toward selecting lower K values.
Another option is to use intraoperative aberrometry. Finally, in case different formulas give different IOL powers, it is better to err toward postoperative myopia, which is easier to correct than hyperopia. Since despite all attempts, it may be difficult to accurately assess anterior and posterior corneal power and astigmatism, postoperative refractive surprises may occur and should be discussed preoperatively with the patient. Negative spherical aberration IOLs benefit patients with previous myopic LASIK. Hyperopic LASIK induces negative spherical aberration and they benefit with traditional spherical IOLs.
For keratoconus and ectasias, I like to regularise the cornea as much as possible preoperatively by using a technique that I have described – Corneal Allogenic Intrastromal Ring Segments (CAIRS). This utilises thin segments of de-epithelialised and de-endothelialised donor corneal stroma that is implanted into mid-peripheral intra-stromal channels similar to synthetic intra-stromal corneal ring segments. This helps to flatten and regularise the cornea, centralise the cone, decrease refractive error and improve uncorrected and best-corrected visual acuity while avoiding synthetic-related complications. Once refractive stability is achieved, cataract surgery may be performed using toric IOLs for residual refractive error. Techniques based on pin-hole optics such as IC-8™ IOL (AcuFocus) or XtraFocus Pinhole implant (Morcher) as well as pin-hole pupilloplasty that can customise the pupil to lie over the visual axis may be used for very high degrees of irregular corneas such as after keratoplasty or radial keratotomy. CAIRS segments may also be tried as a green lasso suture post radial keratotomy.
Infections
A past history of HSV keratitis generally doesn’t indicate anti-viral prophylaxis before and after cataract surgery; however, in rare cases, steroids and surgical trauma may cause reactivation. Postoperatively, topical NSAIDS may be used instead of steroids to decrease this risk. For active corneal ulcers, if performing a therapeutic keratoplasty, the crystalline lens should be retained as a barrier to pathogen entry into the posterior segment.
Ocular surface disease
Dry eye is an important cause for errors in IOL power calculation and can be a source of dissatisfaction, especially with premium IOLs. Prior to biometry, it must be treated with topical lubricants and if required, topical steroids and cyclosporine. I also like to treat blepharitis and Meibomian gland disease prior
to surgery to prevent epithelial toxicity intra- and postoperatively. Cataract surgery by itself can further exacerbate dry eye. Surface-friendly drops should be used and kept to a minimum together with plenty of lubricants.
More severe forms of dry eye such as Sjogren’s syndrome, rheumatoid arthritis, ocular cicatricial pemphigoid (OCP), Stevens-Johnsons syndrome, peripheral ulcerative keratitis (PUK) etc. may develop complications ranging from punctate epithelial keratopathy, filamentary keratitis, peripheral ulcerative keratitis, necrotising scleritis, reactivation of disease, corneal melt etc. Preoperatively, systemic and topical disease should be brought under control and perioperative systemic immunosuppression may
be required.
Surgery should be planned via small clear corneal incisions in OCP and scleral tunnel incisions in PUK. Patients with extremely severe disease such as following chemical burns or with severe limbal stem cell deficiency may, depending on their surface and on the degree of corneal neovascularisation, require Type 1 or Type 2 Boston or LVP keratoprosthesis (KPro). These are combined with cataract surgery if the crystalline lens is still present.
A Type 1 KPro is also required following multiple corneal graft rejections and as mentioned earlier, needs the crystalline lens to be removed. Either an aphakic or pseudophakic Boston KPro may be used depending on IOL status.
Thus, with adequate, judicious planning, preoperative counselling and proper care, many difficult cases with cataract and corneal disease can be given satisfactory outcomes.
Dr Soosan Jacob is Director and Chief of Dr Agarwal’s Refractive and Cornea Foundation at Dr Agarwal’s Eye Hospital, Chennai, India and can be reached at dr_soosanj@hotmail.com
Fig: A- Nucleus emulsification, cortex aspiration and in-the-bag IOL placement performed in an eye with Fuchs’ Endothelial Corneal dystrophy; B- Pre-Descemet’s Endothelial Keratoplasty (PDEK) graft injected into anterior chamber and graft orientation determined using endoilluminator assisted PDEK technique; C- Graft centred and edge folds removed using air pump assisted PDEK technique. Host Descemetic scaffolding seen at inferior 6o’clock position; D- Well placed PDEK graft seen with 80% air fill.
The cornea is the window to the world. It’s a beautiful structure that is also the cataract surgeon’s window to the patient’s eye. It gives a crystal clear, unimpeded, magnified view of the internal structures while acting as the roof to a stable chamber, allowing uneventful surgery. However, we do sometimes come across cataracts in eyes that have some defect in the cornea. In this article, I take a look at some of the problems faced and how
we can manage them.
Fuchs’ Endothelial
Corneal Disease (FECD)
One common situation we sometimes face is the development of cataract in patients with FECD. The questions here of course are – first, whether cataract surgery could worsen the already compromised endothelium and second, if it is better to remove the cataract early when it is soft and requires less phaco energy. If visual loss is not present and the patient is asymptomatic, I generally prefer to advise only observation, sometimes also adding medical management in the form of Rho kinase inhibitors for endothelial regeneration, hypertonic saline to deturgesce the cornea and anti-glaucoma medications to decrease endothelial stress.
If vision starts to decrease or the patient becomes symptomatic, I like to proceed based on grade of FECD, cataract density and estimated contribution to visual loss from each. With minimal endothelial changes and a soft cataract, phacoemulsification can usually be done uneventfully using the least possible phaco energy. I prefer torsional ultrasound and use Arshinoff’s soft shell technique using dispersive and cohesive viscoelastic. I try to debulk the nucleus within the bag before bringing fragments out for iris plane emulsification.
Soft cataracts can be removed easily using high vacuum with very low energy. Dispersive OVD should be intermittently replenished for endothelial coating and wound burns should be avoided. I prefer an extracapsular cataract extraction or manual small-incision cataract surgery under dispersive viscoelastic cover for dense cataracts that would require excessive phaco energy.
Hydrophobic IOL is preferred as hydrophilic IOLs can opacify if air/gas tamponade is required during a possible future endothelial keratoplasty (EK). Preoperative counselling regarding possible slower postoperative recovery as well as possible need for an EK is important even with soft cataracts.
With decompensated FECD, I combine phacoemulsification with in-the-bag IOL placement and an EK irrespective of the grade of cataract.
Though Descemet’s stripping automated EK (DSAEK) and Descemet’s Membrane EK (DMEK) may be performed, my personal preference is pre-Descemet’s EK (PDEK), utilising three techniques that I have described to increase success and repeatability – endoilluminator-assisted PDEK, air pump-assisted PDEK and host Descemetic scaffolding.
I prefer to remove a non-cataractous crystalline lens too as the risk of cataract developing secondary to air tamponade, prolonged steroid usage, inflammation, natural ageing etc. is high and can result in the need for surgery, which can in turn cause loss of precious graft endothelial cells.
Cataract surgery can, however, be challenging in FECD due to poor visibility. Removing the oedematous epithelium can help. Increased stromal oedema whenever the irrigation probe enters the anterior chamber together with any pre-existing haze or minimal stromal scarring decreases visibility in advanced cases. A good quality microscope, capsular staining with trypan blue and use of an endoilluminator as an external oblique light source can help. For a successful EK, it is better to emulsify the cataract working closer to the endothelium than to the posterior capsule to avoid a posterior capsular rent.
Patients with advanced cornea guttata, irido-corneal-endothelial (ICE) syndrome, severe toxic anterior segment syndrome (TASS) and other causes of endothelial dysfunction such as prolonged inflammation or trauma may also be handled using above described principles. Some of these conditions may have peripheral anterior synechiae and glaucoma which may need to be addressed simultaneously. The air pump-assisted PDEK technique allows synechiolysis under air tamponade and prevents bleeding from the iris, hyphema and a fibrinous atmosphere. It helps achieve effective intraoperative graft attachment and therefore gives a greater chance of success even when combined glaucoma surgery is planned.
Scarred cornea
Patients with cataract and stromal and endothelial scarring may require cataract extraction combined with penetrating keratoplasty. This allows better intraoperative visibility, prompt visual rehabilitation after surgery and avoids later cataract extraction and graft endothelial loss. Major disadvantages are inability to accurately estimate IOL power because of unpredictable postoperative keratometry and suture-induced postoperative astigmatism.
Though not very accurate, with experience, it is possible to keep the predicted mean postoperative keratometry within an acceptable range to avoid very large refractive errors. I have found the Maloney intraoperative keratometer very useful to keep suture-related astigmatism to acceptable levels. I also utilise postoperative suture adjustment in the form of selective suture removal or suture replacement for astigmatism that decreases visual acuity.
Cataract extraction may be done open-sky when view is poor. If view permits, closed-chamber phacoemulsification followed by a penetrating keratoplasty can decrease open sky time and allow a rhexis and in-the-bag IOL placement. Phaco incisions should be scleral or limbal and short. Postoperative refractive correction in the form of spectacles or rigid contact lenses may be given.
Postponing cataract extraction to after the keratoplasty allows correction of large refractive errors, but results in endothelial loss at the time of cataract surgery and also requires waiting until complete suture removal resulting in delayed visual rehabilitation.
Corneal dystrophies
and superficial scarring
In case of stromal scarring (not involving Descemet’s-endothelial complex) contributing to a significant decrease in vision, phototherapeutic keratectomy or superficial/deep anterior lamellar keratoplasty (ALK) may be indicated for treatment. I perform this first and defer cataract surgery until after complete suture removal and stabilisation of the refractive error, since endothelial loss is not a major factor.
If, however, the cataract is very dense, depending on extent, level and density of opacity, combined surgery may be done either using techniques that enhance visualisation through scarred corneas or by dissecting the anterior stromal scar by an ALK to a level that allows better visualisation, performing cataract extraction and then proceeding for deeper stromal dissection. If the scar is off the visual axis, or in case of very faint scarring, I perform only cataract surgery.
Irregular cornea
Another situation that occasionally presents is cataract in irregular corneas. This could be secondary to a variety of conditions such as keratoconus, pellucid marginal degeneration, post-LASIK ectasia, radial keratotomy, post-trauma, post-surgery such as after LASIK, keratoplasty, Intacs, cross-linking etc.
In these corneas, when the cataract is soft, I like to do a rigid gas permeable contact lens trial and retinal acuity meter to estimate potential best-corrected visual acuity. Corneal topography is important and multiple measurements should be taken to ensure repeatability. Corneal HOA analysis and pupillometry are also important.
The major problem here is IOL power calculation. In the case of LASIK, IOL power is underestimated post-myopic LASIK and overestimated post-hyperopic LASIK. Post-myopic LASIK, total corneal power measured by ray tracing can be used for better IOL power prediction. Wang-Koch-Hill ASCRS online calculator; Barrett True K No History; No History Shammas-PL; Haigis-L and other methods are useful. Aramberri Double K method corrects effective lens position in SRK/T, Holladay-1 and Hoffer-Q formulas. Hoffer-Savini LASIK IOL power tool allows simultaneous calculation of various formulas to obtain possible K values. Agreement between various K values should be checked while erring toward selecting lower K values.
Another option is to use intraoperative aberrometry. Finally, in case different formulas give different IOL powers, it is better to err toward postoperative myopia, which is easier to correct than hyperopia. Since despite all attempts, it may be difficult to accurately assess anterior and posterior corneal power and astigmatism, postoperative refractive surprises may occur and should be discussed preoperatively with the patient. Negative spherical aberration IOLs benefit patients with previous myopic LASIK. Hyperopic LASIK induces negative spherical aberration and they benefit with traditional spherical IOLs.
For keratoconus and ectasias, I like to regularise the cornea as much as possible preoperatively by using a technique that I have described – Corneal Allogenic Intrastromal Ring Segments (CAIRS). This utilises thin segments of de-epithelialised and de-endothelialised donor corneal stroma that is implanted into mid-peripheral intra-stromal channels similar to synthetic intra-stromal corneal ring segments. This helps to flatten and regularise the cornea, centralise the cone, decrease refractive error and improve uncorrected and best-corrected visual acuity while avoiding synthetic-related complications. Once refractive stability is achieved, cataract surgery may be performed using toric IOLs for residual refractive error. Techniques based on pin-hole optics such as IC-8™ IOL (AcuFocus) or XtraFocus Pinhole implant (Morcher) as well as pin-hole pupilloplasty that can customise the pupil to lie over the visual axis may be used for very high degrees of irregular corneas such as after keratoplasty or radial keratotomy. CAIRS segments may also be tried as a green lasso suture post radial keratotomy.
Infections
A past history of HSV keratitis generally doesn’t indicate anti-viral prophylaxis before and after cataract surgery; however, in rare cases, steroids and surgical trauma may cause reactivation. Postoperatively, topical NSAIDS may be used instead of steroids to decrease this risk. For active corneal ulcers, if performing a therapeutic keratoplasty, the crystalline lens should be retained as a barrier to pathogen entry into the posterior segment.
Ocular surface disease
Dry eye is an important cause for errors in IOL power calculation and can be a source of dissatisfaction, especially with premium IOLs. Prior to biometry, it must be treated with topical lubricants and if required, topical steroids and cyclosporine. I also like to treat blepharitis and Meibomian gland disease prior
to surgery to prevent epithelial toxicity intra- and postoperatively. Cataract surgery by itself can further exacerbate dry eye. Surface-friendly drops should be used and kept to a minimum together with plenty of lubricants.
More severe forms of dry eye such as Sjogren’s syndrome, rheumatoid arthritis, ocular cicatricial pemphigoid (OCP), Stevens-Johnsons syndrome, peripheral ulcerative keratitis (PUK) etc. may develop complications ranging from punctate epithelial keratopathy, filamentary keratitis, peripheral ulcerative keratitis, necrotising scleritis, reactivation of disease, corneal melt etc. Preoperatively, systemic and topical disease should be brought under control and perioperative systemic immunosuppression may
be required.
Surgery should be planned via small clear corneal incisions in OCP and scleral tunnel incisions in PUK. Patients with extremely severe disease such as following chemical burns or with severe limbal stem cell deficiency may, depending on their surface and on the degree of corneal neovascularisation, require Type 1 or Type 2 Boston or LVP keratoprosthesis (KPro). These are combined with cataract surgery if the crystalline lens is still present.
A Type 1 KPro is also required following multiple corneal graft rejections and as mentioned earlier, needs the crystalline lens to be removed. Either an aphakic or pseudophakic Boston KPro may be used depending on IOL status.
Thus, with adequate, judicious planning, preoperative counselling and proper care, many difficult cases with cataract and corneal disease can be given satisfactory outcomes.
Dr Soosan Jacob is Director and Chief of Dr Agarwal’s Refractive and Cornea Foundation at Dr Agarwal’s Eye Hospital, Chennai, India and can be reached at dr_soosanj@hotmail.com