Fundus autofluorescence
Imaging helps to evaluate AMD, macular dystrophies, retinitis pigmentosa and other retinal disorders

Dermot McGrath
Published: Wednesday, May 1, 2019
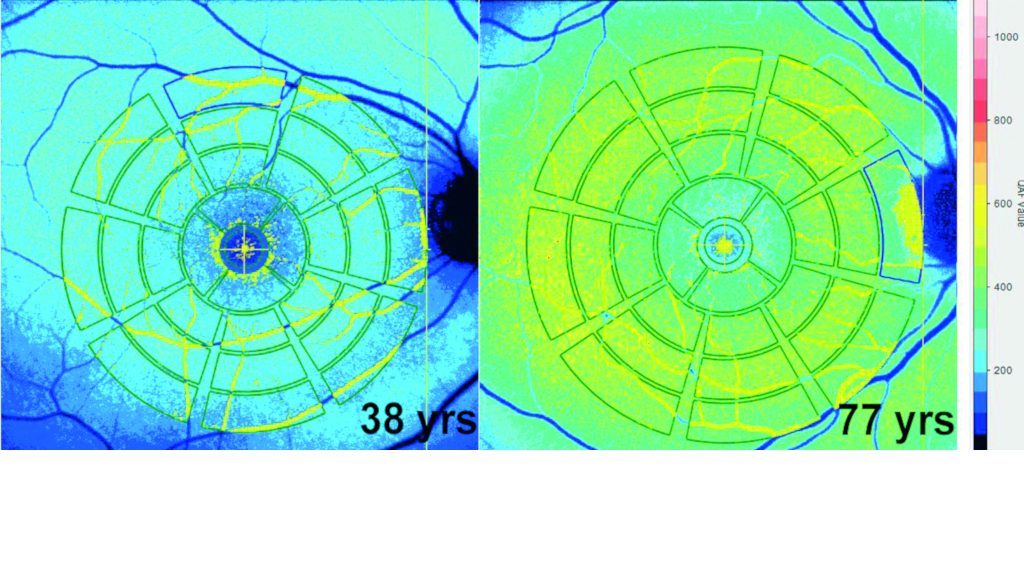 Quantitative autofluorescence measurements allow comparison of AF values among subjects. AF increases with age. Warmer colours indicate higher AF. Courtesy of Thomas Ach MD, FEBO[/caption]
Fundus autofluorescence (FAF) imaging continues to offer a safe, non-invasive, easy-to-perform and reproducible diagnostic method that delivers useful information about retinal health and metabolism, according to Thomas Ach MD, FEBO.
“FAF images are technically easy to acquire, take just a few seconds and are repeatable. They enable us to track patients over a long period and monitor disease progression and are still used as an endpoint in clinical trials,” he told delegates attending the 9th Euretina Winter Meeting in Prague.
Dr Ach explained that FAF uses the autofluorescent properties of tissues, in particular the retinal pigment epithelium (RPE) and its fluorophores. It can be used to evaluate diseases such as age-related macular degeneration (AMD), macular dystrophies, retinitis pigmentosa and various other retinal disorders.
“It gives us a good indication of outer retinal health, with the loss of autofluorescence usually signalling a point of no return for retinal pigment epithelial (RPE) cells,” said Dr Ach, University Hospital of Würzburg, Germany.
Lipofuscin, the term given to fine granules composed of lipid-containing residues of lysosomal digestion (though exact composition is still unknown), is one of the main sources of RPE autofluorescence, said Dr Ach.
“Each RPE cell has hundreds of autofluorescent granules and they show a very characteristic retinal distribution in normal ageing. The exact mechanisms behind the accumulation of lipofuscin are still unclear and there is a lot of debate as to whether it is just a natural marker of age or an actual disease trigger in pathologies such as AMD,” he said.
FAF allows visualisation of different stages of RPE transition in various diseases, from healthy to atrophic in histological slides, said Dr Ach.
Quantitative autofluorescence measurements allow comparison of AF values among subjects. AF increases with age. Warmer colours indicate higher AF. Courtesy of Thomas Ach MD, FEBO[/caption]
Fundus autofluorescence (FAF) imaging continues to offer a safe, non-invasive, easy-to-perform and reproducible diagnostic method that delivers useful information about retinal health and metabolism, according to Thomas Ach MD, FEBO.
“FAF images are technically easy to acquire, take just a few seconds and are repeatable. They enable us to track patients over a long period and monitor disease progression and are still used as an endpoint in clinical trials,” he told delegates attending the 9th Euretina Winter Meeting in Prague.
Dr Ach explained that FAF uses the autofluorescent properties of tissues, in particular the retinal pigment epithelium (RPE) and its fluorophores. It can be used to evaluate diseases such as age-related macular degeneration (AMD), macular dystrophies, retinitis pigmentosa and various other retinal disorders.
“It gives us a good indication of outer retinal health, with the loss of autofluorescence usually signalling a point of no return for retinal pigment epithelial (RPE) cells,” said Dr Ach, University Hospital of Würzburg, Germany.
Lipofuscin, the term given to fine granules composed of lipid-containing residues of lysosomal digestion (though exact composition is still unknown), is one of the main sources of RPE autofluorescence, said Dr Ach.
“Each RPE cell has hundreds of autofluorescent granules and they show a very characteristic retinal distribution in normal ageing. The exact mechanisms behind the accumulation of lipofuscin are still unclear and there is a lot of debate as to whether it is just a natural marker of age or an actual disease trigger in pathologies such as AMD,” he said.
FAF allows visualisation of different stages of RPE transition in various diseases, from healthy to atrophic in histological slides, said Dr Ach.
 Thomas Ach MD, FEBO
Thomas Ach MD, FEBOTags: Fundus autofluorescence
Latest Articles
Making Female Leadership More than a Moment
A remarkable global confluence of women in key positions.
ESCRS Talks Technology at AAO
Europe adopts technological advances, US still waiting for lenses and lasers.
Sorting Out Simultaneous Vision IOLs
The ESCRS Eye Journal Club discuss a new landmark paper on IOL classification and the need for harmonisation of terminology for presbyopic IOLs.
Big Advantages to Small-Aperture IOLs
Small-aperture IOLs offer superior image quality with increased range of focus.
Prioritising Self-Care
Benefits of maintaining physical, emotional, and mental health extend beyond the personal sphere.
Valuing Clinical Trial Design
How inclusivity and diversity can enhance scientific accuracy in research.
Knowing Iris Repair: Using Iridodiathermy in Iris Surgery
Prepare for decentred pupils and uneven irides in multiple situations.
Neuroprotectant Treatment for MacTel Type 2
Intravitreal implant releasing ciliary neurotrophic factor found safe and effective in pivotal trials.