Retina
Gene Therapy in the Real World
Treatment shows safety and effectiveness for patients with rare inherited retinal degeneration.

Gearoid Tuohy
Published: Thursday, March 2, 2023
“ The first ocular gene therapy approved for treating the rare inherited retinal degeneration caused by a bi-allelic RPE65 mutation has shown improvements in both visual function and light sensitivity. “
The two-year interim results following the treatment for LCA2-RPE65 patients provide valuable real-world safety and effectiveness data for voretigene neparvovec (VN; Luxturna®, Spark Therapeutics), according to Dominik Fischer MD, DPhil, FEBOphth.
Voretigene neparvovec is the first ocular gene therapy approved for treating the rare inherited retinal degeneration caused by a bi-allelic RPE65 mutation. The disease causes progressive vision loss and ultimately leads to complete blindness.
Prof Fischer’s real-world study “PERCEIVE” is the first global (ex-US) registry evaluating long-term safety and effectiveness for a gene therapy. PERCEIVE is a prospective, longitudinal, multicentre, multinational, observational project collecting phase IV participants for a 10-year duration. The enrolment period is for 5 years, from 2019 to 2024, with a follow-up period for a further 5 years, from 2024 to 2029. Collected data includes demographic data, adverse events (AEs), medical records, patient-reported outcomes (PROs), caregiver-reported outcomes (CROs), medical history, ocular characteristics, IRD diagnoses, ophthalmic examinations (VA, VF, FST, and OCT), and information on pregnancy outcomes.
The phase III VN data, supported by Spark Therapeutics, showed 31 participants of the pivotal trial randomly assigned to intervention (n = 21) or control (n = 10), leaving a modified-ITT (intention-to-treat) population of 20 intervention and 9 control participants. After 12 months, a mean bilateral multi-luminance mobility test (MLMT) change score reported a value of 1.8 (SD 1.1) in the intervention group versus 0.2 (1.0) in the control group. This primary endpoint’s 1.6 difference (MLMT assay, 95% CI 0·72−2·41, p = 0·0013) led to US FDA approval in December 2017 and EMA approval in November 2018. In addition, the original publication reported no serious, product-related adverse events or deleterious immune responses.i
Following the real-world data for the VN treatment, Prof Fischer showed a total 106 patients enrolled in PERCEIVE by August 2021, 103 patients of which received VN (183 eyes). The mean patient age was 19.5 years, with a 2- to 51-year range. Half of the patients were adults, and half were female. Patients came from 15 countries, the majority of which were from Germany, France, and Italy. Most eyes (80.7%) had an outer nuclear layer present, assessed by OCT. Data recorded on the ellipsoid zone line was intact in 32% eyes, disrupted in 53.9%, and absent in 14 .1%. In addition, the mean BCVA at baseline was 1.14 logMAR, with a range of 0.2 to 2.6 logMAR. Research has not observed any clinically meaningful change in the mean BCVA over time.
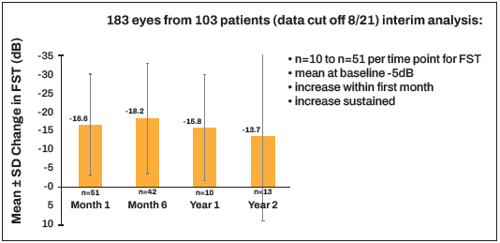
In terms of visual function, assessed by full-field stimulus testing (FST, white light), the mean FST at baseline was -4.56 dB, and its increase had clear changes from month one to year two, showing FST improvements of -16.59 dB, -18.24 dB, 15.84 dB, and 13.67 dB (Figure 1).
“Following the FST, which is a light sensitivity threshold test, almost 100 patients have approximately a 20 dB gain,” Prof Fischer reported, “meaning they are about 10 times more light sensitive now than after the treatment, compared to before, which would be complete night blindness. This nicely reflects what we have seen from the pivotal phase III trials.”
Ocular AEs were reported in 35 patients (34.0%), affecting a total of 50 treated eyes (75 events), with chorioretinal atrophy appearing the most common. However, the benefit for the PERCEIVE study is simply “a numbers game”. Given LCA2- RPE65 is a rare disorder, any individual centre in one or two cities only collects a handful of patients each.
Prof Fischer encouraged clinical investigators to contribute to the data by enrolling their patients in the study.
“We will certainly recognise the contribution, and this will really help us and our patients understand how to prepare for such a treatment.”
Prof Fischer provided this update at the 22nd EURETINA Congress in Hamburg.
Dominik Fischer MD, DPhil, FEBOphth is a consultant ophthalmic surgeon based at the Nuffield Laboratory of Ophthalmology, University of Oxford, UK. Dominik.Fischer@eye.ox.ac.uk
References
i Russell et al, Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial, The Lancet, 2017; 390: 849–860.
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.
Update on Astigmatism Analysis
Is Frugal Innovation Possible in Ophthalmology?
Improving access through financially and environmentally sustainable innovation.
iNovation Innovators Den Boosts Eye Care Pioneers
New ideas and industry, colleague, and funding contacts among the benefits.
José Güell: Trends in Cornea Treatment
Endothelial damage, cellular treatments, human tissue, and infections are key concerns on the horizon.
Making IOLs a More Personal Choice
Surgeons may prefer some IOLs for their patients, but what about for themselves?
Need to Know: Higher-Order Aberrations and Polynomials
This first instalment in a tutorial series will discuss more on the measurement and clinical implications of HOAs.
Never Go In Blind
Novel ophthalmic block simulator promises higher rates of confidence and competence in trainees.