Transplants and AMD
Researchers report promising results with human embryonic stem cell-based tissue transplantation

Cheryl Guttman Krader
Published: Friday, March 1, 2019
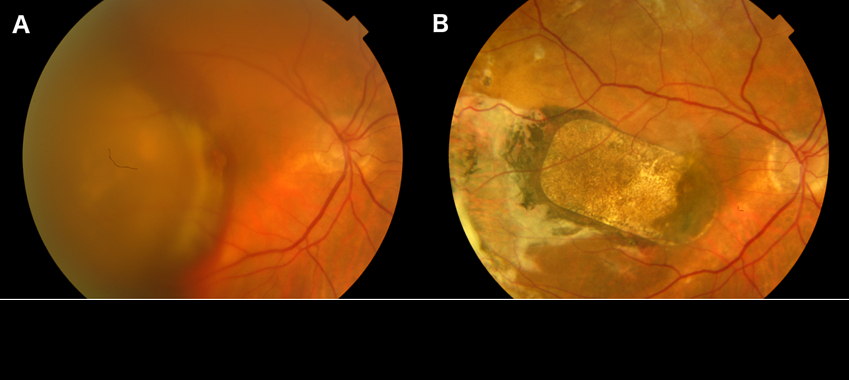 Colour fundus photograph from patient 1. (A) Pre-operatively, and (B) at 18 months after the hESC-RPE implantation[/caption]
Ongoing follow-up of patients in a phase I clinical study supports the safety, feasibility, efficiency and stability of a novel tissue transplantation approach as a regenerative therapeutic strategy for age-related macular degeneration (AMD), reported Dr Odysseas Georgiadis, at the 18th EURETINA Congress in Vienna, Austria.
Speaking on behalf of The London Project to Cure Blindness team, Dr Georgiadis described the procedure, which involves submacular transplantation of a sheet consisting of a fully differentiated, human embryonic stem cell (hESC)-derived retinal pigment epithelium (RPE) monolayer on a coated, synthetic basement membrane. Graft delivery is performed using a purpose-designed surgical tool. He reported encouraging outcomes from follow-up to 18 months in the two patients treated so far.
“Late AMD is considered amenable to cell replacement therapy because it manifests with irreversible cell loss. Although previous surgical approaches, such as macular translocation and autologous RPE transplantation, provided proof of principle for cell replacement, their complexity highlighted the need for an easily accessible cell source and a more feasible surgical paradigm,” said Dr Georgiadis, Moorfields Eye Hospital, London, England.
Colour fundus photograph from patient 1. (A) Pre-operatively, and (B) at 18 months after the hESC-RPE implantation[/caption]
Ongoing follow-up of patients in a phase I clinical study supports the safety, feasibility, efficiency and stability of a novel tissue transplantation approach as a regenerative therapeutic strategy for age-related macular degeneration (AMD), reported Dr Odysseas Georgiadis, at the 18th EURETINA Congress in Vienna, Austria.
Speaking on behalf of The London Project to Cure Blindness team, Dr Georgiadis described the procedure, which involves submacular transplantation of a sheet consisting of a fully differentiated, human embryonic stem cell (hESC)-derived retinal pigment epithelium (RPE) monolayer on a coated, synthetic basement membrane. Graft delivery is performed using a purpose-designed surgical tool. He reported encouraging outcomes from follow-up to 18 months in the two patients treated so far.
“Late AMD is considered amenable to cell replacement therapy because it manifests with irreversible cell loss. Although previous surgical approaches, such as macular translocation and autologous RPE transplantation, provided proof of principle for cell replacement, their complexity highlighted the need for an easily accessible cell source and a more feasible surgical paradigm,” said Dr Georgiadis, Moorfields Eye Hospital, London, England.
 Odysseas Georgiadis
Odysseas Georgiadis