Retina, EURETINA
Targeting the Kallikrein-Kinin System to Treat DME
Novel oral plasma kallikrein inhibitor shows promise in early clinical trial.

Cheryl Guttman Krader
Published: Monday, March 3, 2025
Results of a phase 2a study investigating RZ402 treatment for diabetic macular oedema (DME) show that the novel, orally administered plasma kallikrein inhibitor (PKI) had a favourable safety profile and potential efficacy based on measured change in central subfield thickness (CST), reported Arshad M Khanani MD.
“Systemic therapy for DME holds particular interest because it offers an opportunity for non-invasive bilateral treatment and could be considered as an early intervention to support prevention or treatment of DME,” said Dr Khanani.
“RZ402 is the first PKI and first oral therapy of any class to demonstrate CST improvement in eyes with DME. The phase 2a study results support advancement of RZ402 into longer duration phase 2b/3 studies.”
Interest in targeting the kallikrein-kinin system (KKS) stems from the knowledge diabetes causes injury to retinal blood vessels—which leads to vascular permeability, inflammation, and coagulation—and understanding the KKS is the first line of defence against vascular injury. Preclinical and clinical data also implicates the KKS as a VEGF-independent cause of DME.
“Failures with investigational intravitreal PKI treatment for DME may be explained by failure to reach the vascular target,” Dr Khanani said.
The phase 2a study of RZ402 randomised 94 patients to treatment with placebo or RZ402 50 mg, 200 mg, or 400 mg. Patients took their assigned treatment once daily for three months and were then followed for an additional month once off treatment.
Eligible patients had ETDRS BCVA ≤78 letters and met CST thresholds (males, >320 microns; females, >305 microns). Mean CST at baseline was 408 microns in the placebo group and about 30 to 50 microns higher in the RZ402 groups. Otherwise, mean baseline characteristics were well-balanced across the study groups.
Safety was a primary endpoint, and the collected data showed that adverse events were generally mild and occurred at a similar incidence in the placebo and RZ402 groups. No serious adverse events were judged as related to the study drug, no adverse events associated with other PKIs, and no remarkable changes in electrocardiography, vital signs, or safety laboratory tests.
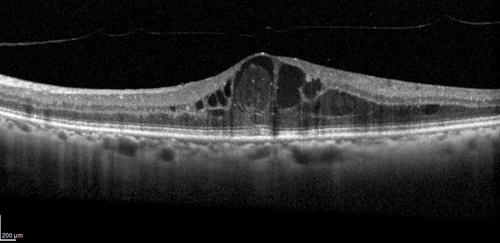
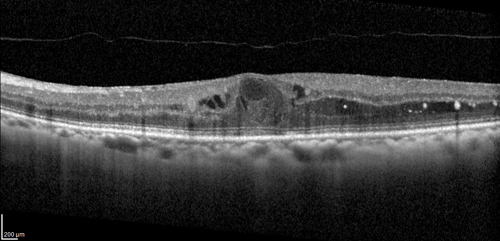
Change in CST was also analysed as a primary endpoint and showed clinically significant reductions in the RZ402 groups compared to controls. After three months, CST increased by a mean of 3 microns in the placebo group and decreased by a mean of 21 to 47 microns in the RZ402 groups, although not in a dose-response fashion. More RZ402 benefit was seen in the subgroup analysis of patients with baseline CST ≥400 microns without a dose-response effect.
“The lack of a dose response likely can be explained by drug saturation of PKI activity past the 200 mg dose,” Dr Khanani said.
BCVA was investigated as a secondary endpoint, but did not show a benefit of RZ402.
“We know that BCVA does not always correlate with CST and fluctuates in patients with DME. In fact, there was an early anomalous increase in the placebo group,” Dr Khanani said.
“However, there was a trend for some improvement over time in the RZ402 groups, and we hope that we will see it has a functional benefit in a longer study.”
Dr Khanani spoke at EURETINA 2024 in Barcelona.
Arshad M Khanani MD, MA is principal investigator of the phase 2a study, managing partner and director of clinical research, Sierra Eye Associates, and clinical professor of ophthalmology, University of Nevada, both of Reno, US. arshad.khanani@gmail.com