Retina
Privosegtor Shows Promise for AON
Improved low contrast visual acuity and reduced retinal ganglion cell loss seen in patients with acute optic neuritis.

Cheryl Guttman Krader
Published: Monday, November 3, 2025
An investigative neuroprotective agent, privosegtor (OCS-05), appears to be promising as an add-on to standard of care treatment for acute optic neuritis (AON), a recent clinical study suggests.
The drug met its primary safety endpoint and key secondary efficacy endpoints assessing structure and function in the phase 2 ACUITY trial, reported Sophie Bonnin MD, PhD.
“The results in ACUITY provided positive proof of concept of a neuroprotective effect for this new class of drug, and its promising benefits for protecting vision and anatomy in AON suggest it could have benefits in multiple other conditions,” Dr Bonnin said.
Privosegtor (OCS-05) is a small molecule peptoid that penetrates the blood-brain and retinal barriers and activates neurotrophic factors. Data from various in vivo preclinical models validated preservation of neurons and axons in animals treated with privosegtor. Because AON is a very good model for neuroprotection and is an indication with no approved treatment, it was chosen as the target for the first clinical trial, Dr Bonnin said.
ACUITY was a double-masked study conducted at four tertiary care neurology and ophthalmology centres in France. Eligible patients had unilateral AON with onset of visual loss symptoms within 12 days prior to randomisation.
Thirty-six patients were randomised to once daily intravenous infusions of privosegtor or placebo in addition to standard of care steroid treatment. The infusions were given on five consecutive days, and patients were followed for six months after the last infusion.
The treatment groups were well-balanced in their baseline demographic and clinical characteristics. Consistent with the epidemiology of AON, the patients were young (mean age approximately 33 years), predominantly female, and 61% had multiple sclerosis. 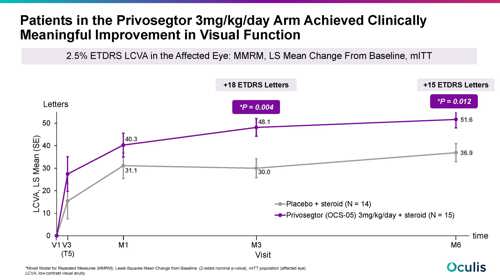
Dr Bonnin presented results for 33 patients comprising a modified intent-to-treat cohort that included all patients who received at least one dose of their assigned study treatment. Cardiac safety was the primary endpoint in this first clinical trial, and the data analyses showed there were no between-group differences in shifts of electrocardiogram parameters. Adverse events were predominantly mild, transient, and not clinically significant. There were no drug-related serious adverse events and no adverse events leading to treatment or study withdrawal.
Data on changes from baseline in ganglion cell/inner plexiform layer (GCIPL) thickness and peripapillary retinal nerve fibre layer (RNFL) thickness, as measured by optical coherence tomography, indicated privosegtor was protecting neurons and axons, respectively. At month three, GCIPL thinning was 43% lower in patients treated with privosegtor versus controls, and RNFL thinning was 28% lower in the privosegtor group. These differences were maintained at month six, Dr Bonnin reported.
Commenting on functional outcomes, she said, “We were very happy to see that the anatomic protection translated to functional benefits for patients.”
Function was assessed by measuring 2.5% low contrast visual acuity (LCVA). The results showed a clinically meaningful improvement from baseline to month three in the privosegtor group (+48.1 ETDRS letters) that was maintained at month six with a difference in LCVA improvement versus placebo of 18 letters at month three and 15 letters at month six (nominal P values for treatment group differences -0.004 and 0.012, respectively).
Dr Bonnin presented at EURETINA 2025 in Paris.
Sophie Bonnin MD, PhD is Deputy Head of the Retina Department at the Rothschild Foundation Hospital, Paris, France. soph.bonnin@gmail.com