Cornea
Knowing Iris Repair: Iris Repair with Prosthetic Devices
New devices on the market expand available options when iris tissue is insufficient.

Soosan Jacob
Published: Monday, March 3, 2025
The previous article in this series discussed aniridia and suture repair of residual iris in partial aniridia. Here, we will discuss complete aniridia and partial aniridia management when the iris tissue is insufficient or too fragile/fibrotic to be sutured or when the surgeon prefers to use a prosthetic iris device (PID) for treating structurally missing iris or functional iris defects such as a fixed, dilated pupil.
PIDs may also be used in conditions like coloboma, albinism, and visually disturbing acquired iris transillumination defects secondary to various causes. Aniridic eyes of any aetiology as well as those with iris pigment deficiency may have comorbidities in other ocular structures and may need combined/multiple surgeries. Some eyes have multiple factors contributing to decreased visual potential, and such complex eyes have a higher risk of postoperative complications. However, prosthetic iris devices still provide decreased photophobia, less glare, better cosmesis, and psychological benefits in these patients.
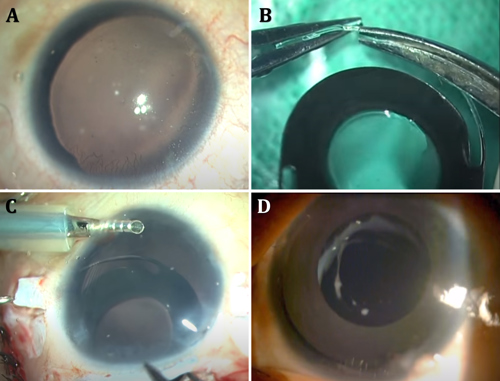
Surgical correction of complete aniridia
The aniridia IOL from Morcher and IO-Care consists of a rigid single-piece PMMA IOL with an overall diameter of 12.50 to 13.75 mm. The optic is about 9.00 to 10.00 mm in diameter, with a clear central area of 3.00 to 5.00 mm and an opaque peripheral black rim and is available with dioptric power as well as plano. The C-shaped haptics may be placed in the sulcus if sufficient iris exists to prevent anterior movement, but they also have eyelets for suture fixation. The haptics may also be externalized via two sclerotomies created under partial thickness—the scleral flaps placed diametrically opposite each other and tucked into intrascleral Scharioth tunnels in a glued IOL technique. The eyelets are trimmed with Vannas scissors before tucking into the tunnel. Advantages include the ability to correct aphakia and aniridia simultaneously.
Disadvantages of this approach include the need for a large incision and decreased intraocular manoeuvrability because of the large and rigid structure. A peripheral aniridic gap may exist beyond the IOL optic in patients with no residual iris. Lack of colour options also decreases usability. Surgeons should monitor the IOL for pigment leaching and inflammatory deposits.
Ophtec had similar aniridia IOL in brown, blue, and green colours, but the untextured, flat diaphragm and limited colour options also decreased the match to the other eye. They announced its discontinuation of sales in 2020 and commenced with the Reper acrylic artificial iris (described below) instead.
The CustomFlex artificial iris (HumanOptics) received FDA approval in 2018. With a pupil size of 3.35 mm and made of silicone, the device is custom-printed/hand-painted to match the contralateral iris in colour, pigment pattern, and distribution, providing a more natural and aesthetic alternative. After measuring the sulcus-to-sulcus width with an intraocular ruler, the CustomFlex is cut with a trephine to the desired overall diameter, then folded and injected through a phaco incision and placed in the ciliary sulcus or within the bag.
Cost, difficulties in sizing, and the absence of a built-in IOL are limitations with this device. However, in eyes with no capsular support, the haptics of a three-piece foldable IOL can be passed through the CustomFlex iris and the implanted IOL using a glued IOL technique, thus giving good support to the artificial iris while providing optical rehabilitation at the same time.
The Custom artificial iris (Reper), made of newer hydrophobic acrylic material, allows folding and injection through small 2.60 mm incisions and is available in many shades. Different models allow centration in the sulcus using haptics, suture fixation, or in-the-bag placement. They are also available with a central optic with dioptric power.
Possible complications for both types of custom artificial iris include IOP spikes and residual iris retraction syndrome.
Cosmetic iris implants
NewColorIris® (Kahn Medical Devices) and BrightOcular® implants (Stellar Devices) were used for cosmetic purposes. The former was withdrawn due to the frequency and severity of sight-threatening complications. The latter was recently reported to have significant complications ranging from uveitis, angle closure glaucoma, corneal decompensation, iris atrophy, and pupillary abnormalities. They were implanted bilaterally in otherwise normal phakic eyes for iris colour change purely for cosmetic reasons and not for any functional or structural iris pathology or for improving vision.
Another category of implant consists of a capsular tension ring (CTR) incorporating a segmental or sectoral iris diaphragm implanted into the capsular bag through the standard phaco incision. Two designs are available from Morcher. The first consists of a black, opaque CTR with a segmental plate-like extension towards the pupil, available in varying arc lengths from small segments used to cover small iris defects to arcs of almost 180 degrees. Two such 180-degree implants can be inserted into the capsular bag to correct complete aniridia, while a single segment can be used to cover large iris defects.
The second design has multiple occluder fins (with gaps in between) and is implanted as a set of two CTRs into the capsular bag before rotating so the fins interdigitate. Both designs are available in varying lengths of central fin extension so surgeons can choose the size of the final pseudopupil form between certain fixed models. These carry the risks of brittleness and breakage. They also need to be carefully rotated into the correct position, which can sometimes be difficult.
Ophtec also has non-CTR devices with standard combinable elements of two basic designs—single and double elements—to create a prosthetic iris.
Other approaches
Corneal tattooing (or keratopigmentation) has been used to treat glare. Tattoo pigment is applied superficially or within a circular intralamellar femtosecond laser channel that is created centred on a pupil with an optic zone of 5.00 to 6.00 mm and extending peripherally to the largest zone possible. It is also very useful for light scatter from small sectoral iris defects or to cover a symptomatic peripheral iridectomy. Intracorneal stromal implants have been postulated to be of use. For patients not desiring surgery, coloured contact lenses or tinted/photochromatic glasses and wide-brimmed hats or baseball caps are other options.
Dr Soosan Jacob is Director and Chief of Dr Agarwal’s Refractive and Cornea Foundation at Dr Agarwal’s Eye Hospital, Chennai, India, and can be reached at dr_soosanj@hotmail.com.