Gene Therapy Progress
Optimism for studies of gene therapy of choroideremia and X-linked retinitis pigmentosa.

Leigh Spielberg
Published: Wednesday, December 4, 2019
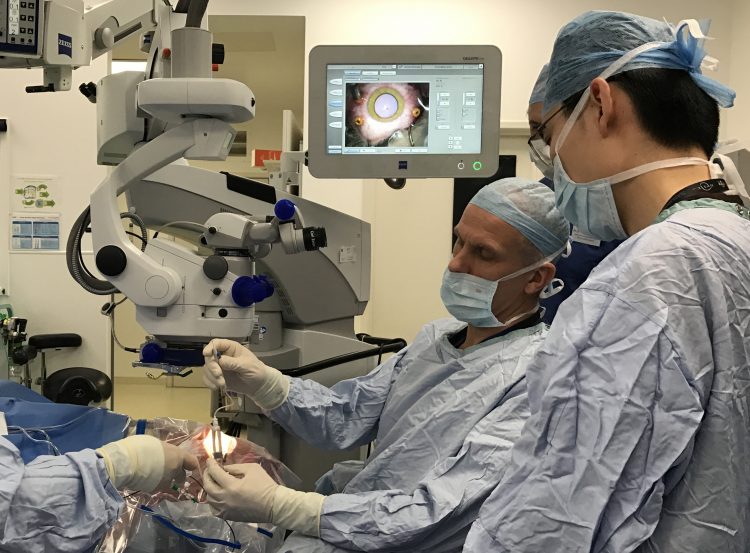 Surgeons prepare to inject a viral vector into the back of the eye. Image courtesy of Robert E. MacLaren FRCOphth, FRCS
Early clinical results with gene therapy for choroideremia and X-linked retinitis pigmentosa suggest there is reason to be optimistic about this therapeutic approach to genetic retinal disease, according to Robert E. MacLaren FRCOphth, FRCS, speaking at the 19th Annual EURETINA Congress in Paris, France.
Dr MacLaren, University of Oxford, presented an update on the current state of the art during a session on the present and future of retinal gene therapy.
Choroideremia is an X-linked recessive chorioretinal dystrophy caused by REP1 protein deficiency, which usually begins with an impairment of night vision and leads to constriction of the peripheral visual field, progressing from annular scotomas to concentric visual field loss. It is characterised by progressive degeneration of the choroid, retinal pigment epithelium and retina.
The choroideremia phenotype is driven by the RPE degeneration. Evidence that it occurs secondary to RPE cell death was revealed when a dominant mutation in RPE65 identified by whole-exome sequencing was shown to cause retinitis pigmentosa with choroidal involvement.
Dr MacLaren first reviewed the technical details of gene delivery for retinal disease.
“The procedure starts with a vitrectomy to gain access to the retina. Intraoperative OCT is then used to guide a measured subretinal injection of balanced salt solution into the potential space between retina and RPE. This detaches the retina, allowing for subsequent subretinal injection of 0.1ml of viral vector suspension into the BSS-bleb,” he explained.
But how high should the vector concentration be?
“The dosing of gene therapy is still relatively unexplored. A suboptimal dose of the vector leads to insufficient numbers of transfused cells, which will die due to the natural history of the disease. However, a toxic dose can also lead to cell death,” said Dr MacLaren.
Finding just the right concentration is needed, and dosing strategies will be the focus of later studies, he added.
He explained that in the meantime, finding patients can be challenging, because the genetics of retinal degenerations aren’t always very straightforward. For example, in a case of X-linked retinitis pigmentosa, with more than 100 retinal degeneration genes to analyse and exome sequencing negative for specific several genes such as RP2 and OFD1, family trees must be consulted.
“In choroideremia, potential patient’s female family members can be screened for carrier status. A ‘typical’ female carrier has had slightly reduced vision since childhood, a mother with poor night vision and a great grandfather who had gone blind due to what was (mis)diagnosed as glaucoma,” he explained, noting that one must be absolutely certain that the phenotype observed in the clinic is caused by the genetic abnormality discovered in the lab, otherwise there will, of course, be no effect.
Choroideremia is in many respects the ideal model for gene therapy in other retinal diseases.
These diseases require early intervention before the onset of visual loss, as it is much easier to prevent a retina from degenerating, via gene therapy, than to reconstruct it once the damage has been done. Considering the success of the current studies, Dr MacLaren was justifiably optimistic about the future of gene therapy for choroideremia.
Big pharma has been taking note of the gene therapy successes. Spark’s LUXTURNA, the first gene therapy for retinal degeneration, paved the way with its approval, but the reimbursements for novel gene therapies are still unclear and payment terms will need to be determined.
In contrast to the relatively slow progression of choroideremia, X-linked retinitis pigmentosa is characterised by its rapid disease progression. Most commonly caused by mutations in the retinitis pigmentosa GTPase regulator (RGPR) gene, X-linked retinitis pigmentosa is also a target of gene therapy.
“The goal is to express RPGR in the photoreceptors,” said Dr MacLaren. “The first human RPGR gene therapy was performed in Oxford Eye Hospital in 2017,” with promising results.
As with choroideremia, the treatment is delivered via an adeno-associated virus (AAV) vector, which is non-enveloped and thus is less likely to induce an inflammatory response. As for the extraocular safety of gene therapy, there are a lot of data suggesting that both short- and long-term results are safe.
Robert MacLaren: enquiries@eye.ox.ac.uk
Surgeons prepare to inject a viral vector into the back of the eye. Image courtesy of Robert E. MacLaren FRCOphth, FRCS
Early clinical results with gene therapy for choroideremia and X-linked retinitis pigmentosa suggest there is reason to be optimistic about this therapeutic approach to genetic retinal disease, according to Robert E. MacLaren FRCOphth, FRCS, speaking at the 19th Annual EURETINA Congress in Paris, France.
Dr MacLaren, University of Oxford, presented an update on the current state of the art during a session on the present and future of retinal gene therapy.
Choroideremia is an X-linked recessive chorioretinal dystrophy caused by REP1 protein deficiency, which usually begins with an impairment of night vision and leads to constriction of the peripheral visual field, progressing from annular scotomas to concentric visual field loss. It is characterised by progressive degeneration of the choroid, retinal pigment epithelium and retina.
The choroideremia phenotype is driven by the RPE degeneration. Evidence that it occurs secondary to RPE cell death was revealed when a dominant mutation in RPE65 identified by whole-exome sequencing was shown to cause retinitis pigmentosa with choroidal involvement.
Dr MacLaren first reviewed the technical details of gene delivery for retinal disease.
“The procedure starts with a vitrectomy to gain access to the retina. Intraoperative OCT is then used to guide a measured subretinal injection of balanced salt solution into the potential space between retina and RPE. This detaches the retina, allowing for subsequent subretinal injection of 0.1ml of viral vector suspension into the BSS-bleb,” he explained.
But how high should the vector concentration be?
“The dosing of gene therapy is still relatively unexplored. A suboptimal dose of the vector leads to insufficient numbers of transfused cells, which will die due to the natural history of the disease. However, a toxic dose can also lead to cell death,” said Dr MacLaren.
Finding just the right concentration is needed, and dosing strategies will be the focus of later studies, he added.
He explained that in the meantime, finding patients can be challenging, because the genetics of retinal degenerations aren’t always very straightforward. For example, in a case of X-linked retinitis pigmentosa, with more than 100 retinal degeneration genes to analyse and exome sequencing negative for specific several genes such as RP2 and OFD1, family trees must be consulted.
“In choroideremia, potential patient’s female family members can be screened for carrier status. A ‘typical’ female carrier has had slightly reduced vision since childhood, a mother with poor night vision and a great grandfather who had gone blind due to what was (mis)diagnosed as glaucoma,” he explained, noting that one must be absolutely certain that the phenotype observed in the clinic is caused by the genetic abnormality discovered in the lab, otherwise there will, of course, be no effect.
Choroideremia is in many respects the ideal model for gene therapy in other retinal diseases.
These diseases require early intervention before the onset of visual loss, as it is much easier to prevent a retina from degenerating, via gene therapy, than to reconstruct it once the damage has been done. Considering the success of the current studies, Dr MacLaren was justifiably optimistic about the future of gene therapy for choroideremia.
Big pharma has been taking note of the gene therapy successes. Spark’s LUXTURNA, the first gene therapy for retinal degeneration, paved the way with its approval, but the reimbursements for novel gene therapies are still unclear and payment terms will need to be determined.
In contrast to the relatively slow progression of choroideremia, X-linked retinitis pigmentosa is characterised by its rapid disease progression. Most commonly caused by mutations in the retinitis pigmentosa GTPase regulator (RGPR) gene, X-linked retinitis pigmentosa is also a target of gene therapy.
“The goal is to express RPGR in the photoreceptors,” said Dr MacLaren. “The first human RPGR gene therapy was performed in Oxford Eye Hospital in 2017,” with promising results.
As with choroideremia, the treatment is delivered via an adeno-associated virus (AAV) vector, which is non-enveloped and thus is less likely to induce an inflammatory response. As for the extraocular safety of gene therapy, there are a lot of data suggesting that both short- and long-term results are safe.
Robert MacLaren: enquiries@eye.ox.ac.uk