Glaucoma research
Research in neurophysiology suggests new targets for neuroprotection in glaucoma
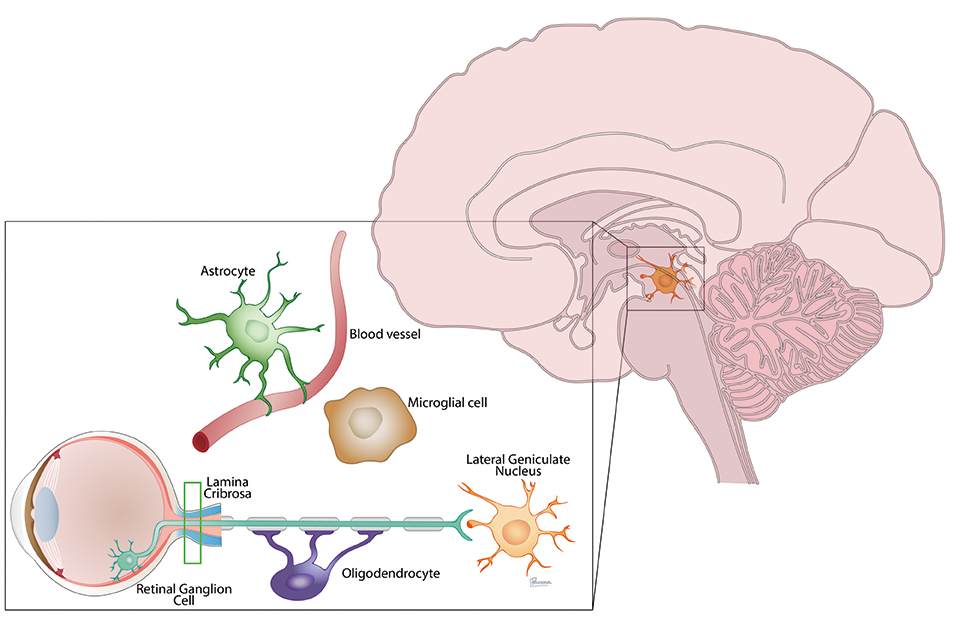
Neural injury in glaucoma extends from the eye to the brain and involves many cell players
While the search for glaucoma treatments typically focuses on the ocular tissues involved with the disease, it now appears that dynamic fluids surrounding the tissues may provide new therapeutic targets, reports Neeru Gupta MD, PhD, MBA, Professor of Ophthalmology and Vision Sciences at the University of Toronto, Canada.
“Right now we really have no cure, and until we can cure the disease we have to be satisfied with somehow slowing progression. And ideally we would use disease-modifying treatments that target the underlying pathology,” she told the 13th European Glaucoma Society Congress in Florence, Italy.
The recent discovery of lymphatic vessels in the uvea raises the possibility of another means of IOP reduction, and also points to some potential new targets for protecting the neurological components of the visual system from glaucomatous degeneration.
She noted that the optic nerve is connected to its target in the lateral geniculate nucleus, and that 90% of the retinal ganglion cells axonal connections go on to the lateral geniculate nucleus and the visual cortex.
Many studies, conducted at centres around the world, have shown that the neural degeneration of glaucoma likewise extends well beyond the optic nerve and into the optic tract, lateral geniculate nucleus, optic radiations ad visual cortex.
“Clearly, neural circuits are destroyed and signal processing is upset and there is a loss of vision. Our targets for rescue are therefore all of these, and there are numerous studies going on that are attempting to target these,” Dr Gupta said.
She noted that there is also evidence that some neurodegenerative diseases like Alzheimer’s disease develop partly as a result of inadequate removal of metabolic waste products, such as amyloid protein, by the glymphatic system. The glymphatic system is an avascular waste clearance pathway for the central nervous system. First described in 2012, its name is a combination of glial and lymphatic (Iliff et al, Science Translational Medicine 2012, 4; 147; DOI:10.1126/scitranslmed.3003748).
Furthermore, she noted that she and her associates have found evidence for cerebrospinal fluid entry into the optic nerve using a glymphatic pathway. In a study in which fluorescent dextran tracers were injected into the cisterna magna in adult mice, CSF was detected in the subarachnoid space surrounding the orbital optic nerve and within the optic nerve itself (Mathieu et al, Invest Ophthalmol Vis Sci. 2017;58: 4784–4791).
“When we think of protecting the structure and function of the optic nerve we are always thinking about the tissues, but it is important to remember all of our tissues are surrounded by fluids. These fluids are very dynamic and full of molecules critical to the tissues' maintenance and survival, and are also essential for the removal of waste,” Dr Gupta explained.
She noted that tracers found within the nerve were specifically localised between isolectin-labelled blood vessels and GFAP-positive astrocytes or aquaporin 4-labelled astrocytic endfeet. That suggests CSF enters the optic nerve though a channel lined by the vascular endothelium on one side and aquaporin 4-labelled glial cells on the other.
“Currently, lowering IOP is a way to protect our optic nerve from damage. It is possible that the glymphatic system of the optic nerve helps to remove waste and may represent a new target for glaucomaa,” Dr Gupta concluded.
EXPANDING THE RANGE OF TREATMENT OPTIONS
There are already several examples of pathology-targeted treatments for different types of glaucomas, such as anti-VEGF treatment for neovascular glaucoma, laser peripheral iridotomy for primary angle-closure glaucoma and cataract removal for phacomorphic glaucoma, she said.
In all types of glaucoma, treatment is based on lowering IOP. And although the treatments do not directly target the neurodegenerative processes that characterise the condition, research shows that they do reduce damage to the optic nerve and also the resulting visual loss.
Research is continuing to expand this line of attack on the disease. The second decade of the current century has seen the introduction of rho-associated protein RHO-kinase inhibitors. Netarsudil (Aerie Pharmaceuticals), the first in this new class of drug, was recently approved by the US FDA, and reportedly lowers IOP by relaxing the cells of the trabecular meshwork, increasing outflow.
DIRECT NEUROPROTECTION
However, actual neuroprotection outside of IOP reduction remains an elusive target. Memantine, an agent used in the treatment of Alzheimer’s disease, showed early considerable promise in a monkey model of glaucoma (Yucel et al., Arch Ophthalmol. 2006 Feb;124(2):217-25; Hare WA, WoldeMussie E, Lai RK, et al., Functional measures. Invest Ophthalmol Vis Sci. 2004;45(8):2625-2639), but failed to reach its endpoint in a randomised prospective controlled clinical trial (Weinreb RN et al., Ophthalmology. 2018 Aug 3. pii: S0161-6420(18)30029-0. doi:10.1016/j.ophtha.2018.06.017. [Epub ahead of print]).
“For the moment there really is no cure for glaucoma, no neuroprotective drug available to date, and I think in order to protect the optic nerve we have to think about it in relationship to the central visual system and all the other cell players,” Dr Gupta said.
She noted, for example, that new data suggests that gene therapy can be used to decrease the oxidative stress in the optic nerve and thereby make it more resistant to damage by elevated IOP. She cited a study in which gene therapy with an adenovirus-associated vector carrying a gene for a type of erythropoietin (rAAV.EpoR76E) preserved visually evoked potentials and axon transport in the DBA/2J mouse model of glaucoma. It appeared to achieve this effect at least in part by decreasing infiltration of peripheral immune cells, modulating microglial reactivity and decreasing oxidative stress (Hines-Beard et al., J Neuroinflammation. 2016; 13: 39).
Neeru Gupta: guptan@smh.ca
Authors
Roibeard O’hEineachain
Published
Wednesday, January 30, 2019
Category