Innovative Implant
Implant replacing 200-700 microns of cornea could reduce global blindness

Howard Larkin
Published: Tuesday, December 24, 2019
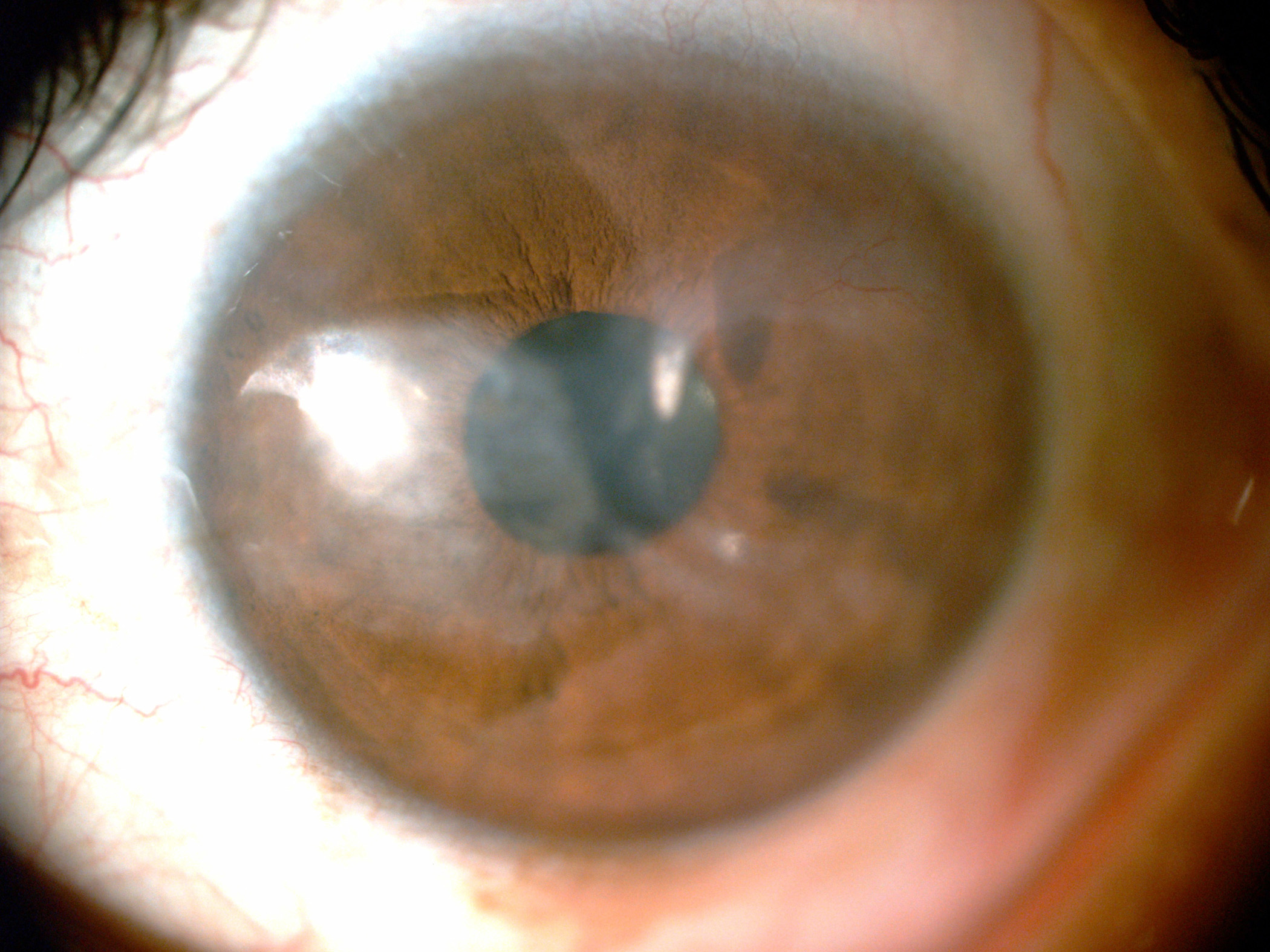 A patient with a corneal scar. Image courtesy of Yichieh Shiuey MD
With approximately 30 million people affected by unilateral corneal blindness and 10 million people by bilateral corneal blindness, corneal opacity is the third leading cause of blindness worldwide. Yet fewer than 200,000 corneal grafts are available annually, meaning less than 1% of patients can receive corneal transplantation. Use of the penetrating Boston keratoprosthesis is also limited by the need for donor tissue and the risk of severe complications.
A recently developed non-penetrating keratoprosthesis could break this mismatch of tissue supply versus demand, Yichieh Shiuey MD told the innovators session at the ASCRS ASOA 2019 Annual Meeting in San Diego, USA.
“It is my sincere hope that this and other technologies will finally be able to rid the world of the scourge of corneal blindness.”
Office procedure
Compared with penetrating alternatives, the minimally invasive KeraKlear (KeraMed) offers easier implantation, equal or better visual outcomes and lower risk of complications, including extrusion, endophthalmitis and glaucoma, said Dr Shiuey, who is KeraMed’s founder and CEO, and the device’s inventor.
A patient with a corneal scar. Image courtesy of Yichieh Shiuey MD
With approximately 30 million people affected by unilateral corneal blindness and 10 million people by bilateral corneal blindness, corneal opacity is the third leading cause of blindness worldwide. Yet fewer than 200,000 corneal grafts are available annually, meaning less than 1% of patients can receive corneal transplantation. Use of the penetrating Boston keratoprosthesis is also limited by the need for donor tissue and the risk of severe complications.
A recently developed non-penetrating keratoprosthesis could break this mismatch of tissue supply versus demand, Yichieh Shiuey MD told the innovators session at the ASCRS ASOA 2019 Annual Meeting in San Diego, USA.
“It is my sincere hope that this and other technologies will finally be able to rid the world of the scourge of corneal blindness.”
Office procedure
Compared with penetrating alternatives, the minimally invasive KeraKlear (KeraMed) offers easier implantation, equal or better visual outcomes and lower risk of complications, including extrusion, endophthalmitis and glaucoma, said Dr Shiuey, who is KeraMed’s founder and CEO, and the device’s inventor.
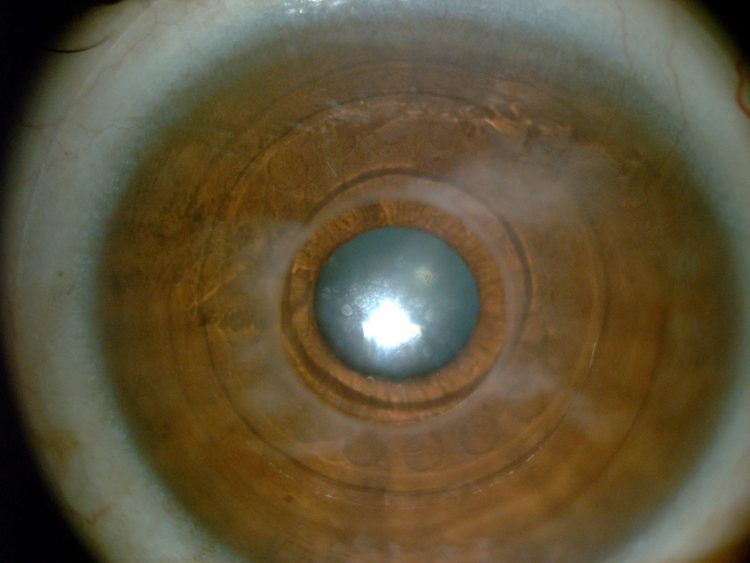 The same patient following implantation of the KeraKlear
Unlike PK and the Boston K-PRO, KeraKlear can be implanted in any clean environment such as an office or laser suite and does not require a trained cornea transplant surgeon or a sterile, specially equipped operating room. The flexible device is placed through a circular 3.5mm partial-thickness incision into a uniform 8.0mm corneal pocket, and can replace 200-to-700 microns of diseased corneal tissue. A femtosecond laser is usually used to cut the pocket and semi-automated pocket-making devices may be used where lasers are not available. However, manual dissection is not a viable option because the human hand cannot create a uniform depth pocket, Dr Shiuey said.
Good outcomes
In a 50-month follow-up of 26 cornea blind patients implanted with KeraKlear, 92% achieved 20/200 or better vision. This level of visual acuity was as good or better than several Boston K-PRO studies and was also similar to the outcomes of several PK studies, Dr Shiuey reported. Unlike corneal transplantation, vision improves immediately after surgery with the KeraKlear, and vision is stable within one-to-two months, he added.
To date, no patient receiving the implant has experienced endophthalmitis, retroprosthetic membrane or increased intraocular pressure or glaucoma. Extrusion, corneal melting and non-endophthalmitis infection rates were also similar or lower than penetrating alternatives. A separate published study of 15 patients reported similar results (Alió JL, et al. Br J Ophthalmol. 2015;99:1483-1487).
The KeraKlear device is commercially available outside the USA, and recruitment for a FDA clinical trial is currently under way at Harvard University, Duke University, University of California, Irvine and the Cincinnati Eye Institute.
Yichieh Shiuey: shiuey@yahoo.com
The same patient following implantation of the KeraKlear
Unlike PK and the Boston K-PRO, KeraKlear can be implanted in any clean environment such as an office or laser suite and does not require a trained cornea transplant surgeon or a sterile, specially equipped operating room. The flexible device is placed through a circular 3.5mm partial-thickness incision into a uniform 8.0mm corneal pocket, and can replace 200-to-700 microns of diseased corneal tissue. A femtosecond laser is usually used to cut the pocket and semi-automated pocket-making devices may be used where lasers are not available. However, manual dissection is not a viable option because the human hand cannot create a uniform depth pocket, Dr Shiuey said.
Good outcomes
In a 50-month follow-up of 26 cornea blind patients implanted with KeraKlear, 92% achieved 20/200 or better vision. This level of visual acuity was as good or better than several Boston K-PRO studies and was also similar to the outcomes of several PK studies, Dr Shiuey reported. Unlike corneal transplantation, vision improves immediately after surgery with the KeraKlear, and vision is stable within one-to-two months, he added.
To date, no patient receiving the implant has experienced endophthalmitis, retroprosthetic membrane or increased intraocular pressure or glaucoma. Extrusion, corneal melting and non-endophthalmitis infection rates were also similar or lower than penetrating alternatives. A separate published study of 15 patients reported similar results (Alió JL, et al. Br J Ophthalmol. 2015;99:1483-1487).
The KeraKlear device is commercially available outside the USA, and recruitment for a FDA clinical trial is currently under way at Harvard University, Duke University, University of California, Irvine and the Cincinnati Eye Institute.
Yichieh Shiuey: shiuey@yahoo.com