Mesenchymal stromal cells (MSCs) and their secreted factors have promising potential for therapeutic use to modulate ocular inflammation or promote ocular surface repair, according to new research presented at the 2021 ARVO virtual conference.
“Clinical trials are just getting started to assess the safety and efficacy of these modalities. Much more work is needed to characterise the key mediators and optimal conditions for their production,” said Ali R Djalilian MD, Professor of Ophthalmology, University of Illinois College of Medicine, Chicago, Illinois, USA.
Dr Djalilian reviewed findings from studies conducted in culture and animal models that demonstrated the immunomodulatory and regenerative effects of MSCs—or “secretome”—and provided an overview of related clinical research.
PRECLINICAL FINDINGS
Dr Djalilian explained that MSCs, found in the cornea and most adult tissues, are suitable for cell-based therapies because they can be isolated and readily grown to billions of cells. Interest in their therapeutic application centres primarily on their immunomodulatory effects. The MSCs also have cytoprotective and trophic effects. Potentially, he said, these multipotent cells could be engrafted into local tissues where they might differentiate into local tissue types.
Preclinical studies investigating the therapeutic potential of MSCs include research by Dr Djalilian and colleagues using an animal model of limbal stem cell deficiency (LSCD). The results showed limbal MSCs delivered in a fibrin gel inhibited both neovascularisation and inflammation and modulated macrophage-mediated inflammation by promoting the M2-like phenotype involved in repair and inflammation resolution. The team’s work also determined that pigment epithelium-derived factor (PEDF) is one of the key mediators MSCs secreted.
Other research focused on use of the MSC secretome, which would offer logistical advantages as a clinical therapeutic agent compared with the MSCs. In a study using the LSCD animal model, Dr Djalilian and colleagues found that topical application of MSC-secreted factors was effective for modulating inflammation, promoting epithelial healing, and helping to restore the corneal phenotype. They also showed in an ex vivo model that treatment with the corneal MSC secretome mitigated corneal endothelial cell loss resulting from phacoemulsification ultrasound-induced injury.
HUMAN TRIALS
Clinical studies have already been done investigating MSCs for ocular surface reconstruction. Spanish researchers published positive results after conducting a proof-of-concept clinical trial investigating whether an MSC transplant could treat eyes with corneal epithelial stem cell deficiency. Other studies are underway in India, France, Denmark, and China, Dr Djalilian said.
Additionally, the team recently launched a phase 1/2 clinical trial that will use cryopreserved allogeneic bone marrow-derived MSCs to treat patients with acute chemical injury or corneal ulcers. The MSCs will be delivered by subconjunctival injection, but the investigators plan to explore alternative routes, including topical MSC administration in fibrin glue and intrastromal injection. The study’s primary objective is to evaluate safety, but the outcomes assessments will also look at epithelial defect closure, stromal healing, and corneal scarring.
Dr Djalilian and colleagues have also designed a phase one clinical trial to investigate the use of MSC secretome eye drops in patients with acute or chronic corneal epithelial disease. Recruitment, set to begin later in 2021, will target patients with partial LSCD and neurotrophic keratitis. Again, safety and tolerability are the primary outcomes, but data to gain insight about potential efficacy in the different pathologies is also being collected.
EXOSOME RESEARCH
There are also preclinical investigations investigating the potential therapeutic use of corneal MSC-derived exosomes. In studies of these membrane-bound extracellular nanovesicles—which act as MSC regenerative factor carriers—Dr Djalilian and colleagues demonstrated that the exosomes were taken up by human corneal epithelial cells in culture and by corneal epithelium in vivo after topical application in an animal wound healing model. Results from an animal study also showed the exosome treatment accelerated corneal epithelial wound healing. Dr Djalilian reported that macrophages from peripheral blood mononuclear cells exposed to the exosomes adopted the inflammation resolving/less angiogenic M2 phenotype promoted by MSCs.
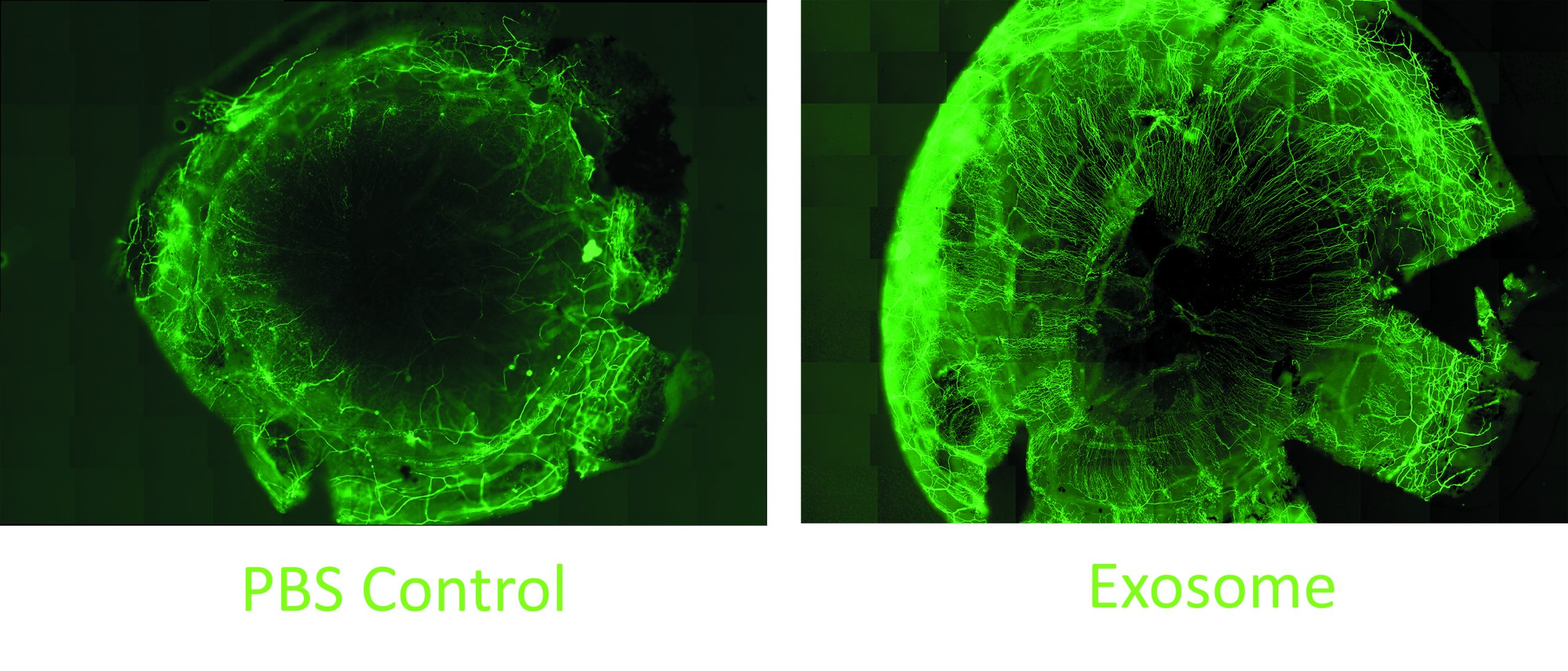
In other research, the team studied the effect of the MSC exosomes on corneal nerves. Their work showed that when added to cultures of trigeminal nerve ganglion cells, the exosomes promoted neurite growth to an extent comparable to that induced by culture supplementation with nerve growth factor. They also showed in an in vivo animal study that topical MSC exosome application significantly accelerated corneal nerve regeneration following mechanical injury.
Another study performed with diabetic mice that had developed loss of corneal nerves showed subconjunctival injection of the exosomes promoted corneal nerve regeneration. The exosome-treated animals demonstrated an increase in corneal sensation.
“These are small pilot studies, but it is encouraging that we are already seeing clear differences between the exosome-treated and control groups,” Dr Djalilian said.
Dr Djalilian acknowledges the funding support from the Department of Defense, National Eye Institute (NIH), and Research to Prevent Blindness for the studies mentioned.
Ali Djalilian: adjalili@uic.edu