Glaucoma
Glaucoma Treatment Paradigm Shift
A focus on the place of canaloplasty

“ If one could intervene before this fibrosis, one can restore natural flow via a restorative/dilating MIGS procedure such as canaloplasty “
By Dr Karl Mercieca
Surgical treatment for glaucoma has dramatically changed within my career as a glaucoma surgeon. Early in my training, the treatment paradigm split between filtration surgery, with trabeculectomy the [most common] procedure, and topical therapy involving various drugs and target mechanisms. Whilst trabeculectomy is no doubt highly effective, it can also carry a substantial complication risk. On the other hand, medications may not be effective enough, and their chronic use can lead to ocular surface disease, exacerbating the very problem they were designed to address. The gap between the two options was extremely wide.
Micro-invasive glaucoma surgery (MIGS) was introduced around 2012 to bridge the gap. And in the last decade, we have witnessed a flourish of new options, roughly split into three areas based on target anatomy: Schlemm’s canal and/or the trabecular meshwork, supraciliary space, and subconjunctival space. Most of these approaches respect the essence of MIGS: minimal trauma with an ab-interno approach, favourable efficacy, a high safety profile when compared to more invasive glaucoma surgery, and rapid recovery.1
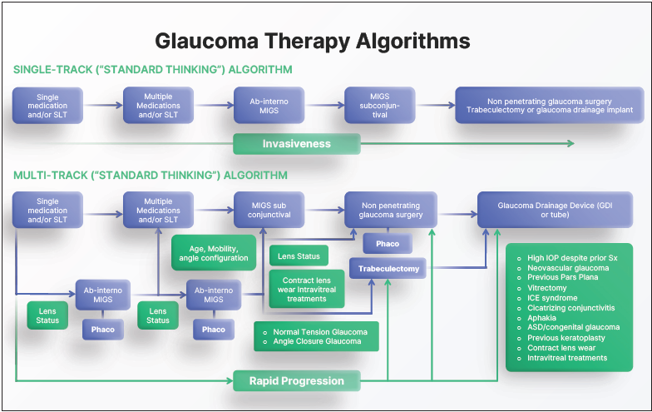
With such variety, there is ongoing debate regarding the criteria for selecting the most appropriate MIGS procedure for each patient. The pathway in Figure 1 (“Single-Track” algorithm) broadly reflects the selection criteria. As with any treatment algorithm, this is subjective to the clinician; no system—including the one depicted in Figure 1—is rigid or linear. However, my clinical and research experiences have shown me a systematic graded approach to glaucoma management allows a safe and reasoned way to care for patients.
Overall, MIGS can be classified into those that cut, stent, and dilate/restore patient trabecular outflow anatomy and physiology—with surgeons sometimes targeting more than one simultaneously. Something exciting about MIGS is the possibility of taking a truly nuanced approach to glaucoma surgery. The first thing to consider is the preoperative features: anatomical eye structure, type of glaucoma, patient-related concerns, medication burden, target pressure, disease comorbidity, rate of progression, and whether treatment will combine with cataract surgery. Moreover, we must consider performing the procedure properly and safely and examine the postoperative follow-up requirements. The variables are many, and it is, at present, challenging to provide a definitive criterion. However, I do believe there are generic guidelines we can recommend and follow.
Canaloplasty first
First and foremost, always think about the disease pathophysiology and elevated pressure, typically found at the trabecular meshwork (TM). The pathology involves abnormal accumulation of extracellular matrix in the TM over time, resulting in chronic inflammation, fibrosis, and sclerosis. If left untreated, the situation becomes irreversible over time; at that point, bypassing the stiff and fibrotic TM with a stent using a procedure such as iStent or Hydrus makes sense. These devices should be in the glaucoma surgeon’s armamentarium, but of course it would be desirable not to have a foreign body in the eye—it is best to work with the natural ocular physiology and restore natural anatomy wherever possible.
Based on my stepwise approach, if one could intervene before this fibrosis, one can restore natural flow via a restorative/dilating MIGS procedure. One such procedure, canaloplasty, combines the effectiveness of pressurised viscodilation and catheterisation over the entire conventional outflow pathway. It works by catheterising Schlemm’s canal, pushing out herniations, and removing outflow resistance in these areas. This step combines with the pressurised injection of a high molecular-weight ophthalmic viscosurgical device (OVD), further stretching the TM trabecular plates, simultaneously dilating Schlemm’s canal and the collector channels.2–4 We still lack a preoperative test allowing TM stiffness and alteration measurement, as well as one showing the degrees of patency along Schlemm’s canal.
Via ab-interno or ab-externo?
Canaloplasty can be performed via an ab-interno approach with two devices: the iTrack Advance microcatheter (Nova Eye Medical, US), which delivers +100 μl of OVD over 360 degrees of the canal; and the OMNI (Sight Sciences, US), which delivers 5.5 μl across 180 degrees and can couple with ab-interno trabeculectomy. Alternatively, in more severe cases, an ab-externo technique where a tensioning suture placed in Schlemm’s canal ensures prolonged aqueous outflow and a higher IOP reduction—offers efficacy approaching that of trabeculectomy and reduces IOP and medication dependence in moderate to severe glaucoma patients while maintaining a good safety profile.5 While both techniques result in outflow system restoration that delivers a significant and sustained IOP reduction and medication burden,6–8 the ab-interno technique preserves conjunctiva and can thus occur earlier, reducing the risk of scarring and failure if a future subconjunctival filtering procedure is needed.
The glaucoma treatment pathway
In using the stepwise but flexible approach depicted in Figure 1 (“Multi-Track” algorithm), I always try to control IOP with the least number of medications, which may mean also offering SLT in some cases. The target IOP is important, but it depends on the many variables affecting the magnitude and rate of glaucoma progression. When drops (with or without SLT) are not enough, particularly if a patient requires more than two medications, ab-interno canaloplasty is the procedure that most closely adheres to the “MIGS principles.” Stenting and/or cutting would subsequently find their place within the algorithm, followed by more invasive bleb-forming procedures.
This diagrammatical treatment algorithm incorporates the many secondary features MIGS has brought to glaucoma surgery. Canaloplasty is not always preferable, namely, when the TM is already fibrotic, when the surgeon expects complicated cataract surgery, or when the glaucoma type is not right. Other factors in selecting a particular MIGS relate to risk analysis and disease magnitude and progression; in some cases, possibly pushing for stronger and consequently more invasive procedures earlier. On the other hand, there are exceptions in favour of canaloplasty—in pseudophakic patients with early glaucoma, where the evidence for microstents is not very strong, ab-interno canaloplasty would be my current first choice.
Conclusion
Every MIGS has its place in the glaucoma paradigm, and its true value is enabling the surgeon to customise the procedure to each patient. To realise the true benefits of each MIGS device, surgeons must carefully consider the preoperative and postoperative characteristics, review the published evidence, and engage in open and ongoing dialogue with their peers to determine patient selection criteria, procedure pearls, protocol suggestions, and best practice.
I also believe greater MIGS awareness among cataract surgeons would greatly help in the interventional management of ocular hypertension and early glaucoma. Coupling some MIGS procedures, such as ab-interno canaloplasty, more often when these patients undergo cataract removal could be an important step in preventing glaucoma development or progression—and may delay or avoid more invasive procedures. For citation notes, see page 46.
Karl Mercieca MD, PGCME, FRCOphth, FEBOS-GL currently works as a senior consultant ophthalmic surgeon and is Head of the Glaucoma Secto r at the University of Bonn Eye Clinic in Bonn, Germany. Karl.Mercieca@ukbonn.de.
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
The 5 Ws of Post-Presbyopic IOL Enhancement
Fine-tuning refractive outcomes to meet patient expectations.
AI Shows Promise for Meibography Grading
Study demonstrates accuracy in detecting abnormalities and subtle changes in meibomian glands.
Are There Differences Between Male and Female Eyes?
TOGA Session panel underlined the need for more studies on gender differences.
Simulating Laser Vision Correction Outcomes
Individualised planning models could reduce ectasia risk and improve outcomes.
Need to Know: Aberrations, Aberrometry, and Aberropia
Understanding the nomenclature and techniques.
When Is It Time to Remove a Phakic IOL?
Close monitoring of endothelial cell loss in phakic IOL patients and timely explantation may avoid surgical complications.
Delivering Uncompromising Cataract Care
Expert panel considers tips and tricks for cataracts and compromised corneas.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.