Glaucoma
CID: A New Pathway for Glaucoma Surgery
Novel cilioscleral device shows promising results for IOP management in treating open- and narrow-angle glaucoma.
“ The 42 patients implanted with the CID in clinical studies had a mean IOP lowering of 32% at year two, with a measured 16.2 mmHg (range 12.0 to 20.0 mmHg) pressure average. “
Timothy Norris reports
Encouraging clinical results in the two first-in-human SAFARI 1 and SAFARI 2 studies in open-angle glaucomas (Shaffer 3–4) showed Cilioscleral Interposition Device (CID) safety and efficacy, promising new standards for the future of IOP management and glaucoma surgery.
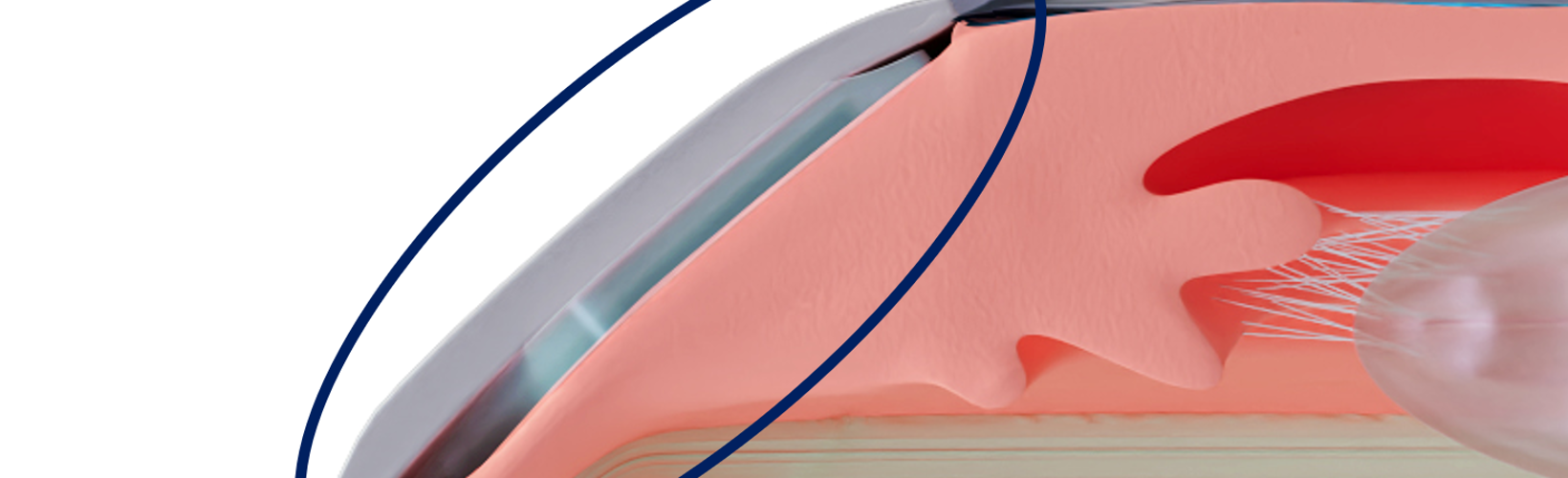
The CID is a 25% hydrophilic acrylic, 6.0 by 4.0 mm, 200 μm thick device surgically inserted 2.0 mm behind the limbus through two scleral incisions. For the first time, a glaucoma implant is intended to lower IOP without entering the anterior chamber or creating subconjunctival filtration.
“It is a very low-risk device. Placed ab externo between the sclera and the ciliary body, it opens an uveoscleral pathway between the two tissues, draining the aqueous humour directly from the uveal trabecular meshwork towards the supraciliary space,” said Ciliatech’s co-founder and medical director Dr Philippe Sourdille, who added the operation is performed under topical anaesthesia.
The 42 patients implanted with the CID in clinical studies had a mean IOP lowering of 32% at year two, with a measured 16.2 mmHg (range 12.0 to 20.0 mmHg) pressure average. The consistent year-one results presented at the ESCRS iNovation Day 2022 were confirmed in year two.
“This is a very important milestone because many promising devices usually fail to deliver on their promises at this point,” Dr Sourdille said. “This device could change the surgical approach to glaucoma.”
The two-year outcomes from the SAFARI 1 and SAFARI 2 studies will be officially presented at the 41st ESCRS Congress in Vienna, though more research and development are underway to refine this implant further.
“Following a slight modification to the implant design, we launched the SAFARI 3 trial in June 2022, which has 44 patients enrolled,” he explained. “The two previous studies considered only open-angle glaucoma; this time, half of SAFARI 3 patients have narrow-angle glaucoma (Shaffer 1–2).”
And so far, the preliminary results are promising. “Not only were we able to achieve a 1.6 mmHg lower intraocular pressure compared to the SAFARI 1 and 2 mean values at six months, but the same IOP lowering was present in the narrow-angle group. This is the most interesting finding about this study so far,” Dr Sourdille said.
“I can only speak for the first six months, but we are very encouraged because these are very good numbers, and I am confident we will be able to widen our field of indications to narrow-angle glaucoma as well.”
Possible option for narrow-angle glaucoma
The CID could become an especially important option for glaucoma surgeons dealing with narrow-angle glaucoma when the gonioscopy-assisted ab interno approach is not possible—and the lens does not need to be removed to lower the IOP.
No operations have been needed thus far in the series, but the CID can easily combine with other surgical techniques and devices for treating glaucoma. Should it be indicated, filtrating or non-filtrating operations, such as trabecular stents (with ab interno and ab externo approach), could be performed without a loss of chance for the patients. A cyclodialysis implant could also be used in a different surgical site.
This is made possible, he said, by optically sparing the conjunctiva and the anterior segment of the eye using only two radial conjunctival incisions over the scleral incision sites. And the CID location neither prevents access to the corneoscleral trabeculum nor the subconjunctival filtration.
CID implantation takes no longer than 15 minutes. The implantation requires the surgeon to perform two manual penetrating scleral incisions. They must apply scleral sutures after the implantation to avoid any subconjunctival bleb, he explained.
“I can tell from the experience of Dr Lilit Albert Voskanyan, Chief of Glaucoma Surgery at the Malayan Ophthalmological Center in Yerevan—who conducted and performed the three series—that her maximum time for the implantation of a CID device is set around nine minutes. By doing just two radial conjunctival incisions, this surgical technique minimises the risk of conjunctival scarring and the failure risk of a second operation.”
Ciliatech hopes to apply for the CE mark before the end of the year or possibly next year.
“We are already consulting with the FDA to define the best approach to obtain US market approval,” Dr Sourdille concluded.
Philippe Sourdille MD is an ophthalmologist, researcher, inventor, and medical director and co-founder of Ciliatech in Chavanod, France. p.sourdille@cilia.tec
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
The 5 Ws of Post-Presbyopic IOL Enhancement
Fine-tuning refractive outcomes to meet patient expectations.
AI Shows Promise for Meibography Grading
Study demonstrates accuracy in detecting abnormalities and subtle changes in meibomian glands.
Are There Differences Between Male and Female Eyes?
TOGA Session panel underlined the need for more studies on gender differences.
Simulating Laser Vision Correction Outcomes
Individualised planning models could reduce ectasia risk and improve outcomes.
Need to Know: Aberrations, Aberrometry, and Aberropia
Understanding the nomenclature and techniques.
When Is It Time to Remove a Phakic IOL?
Close monitoring of endothelial cell loss in phakic IOL patients and timely explantation may avoid surgical complications.
Delivering Uncompromising Cataract Care
Expert panel considers tips and tricks for cataracts and compromised corneas.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.