Cornea
Beyond PK
What lies ahead for corneal endothelial disease treatment?

Soosan Jacob
Published: Monday, May 1, 2023
Dr Soosan Jacob Reports
Currently, eye banks can meet only 1.5% of the global keratoplasty demand for donor corneas. Researchers around the world have taken this as a challenge to develop innovative approaches that could help make the most of the existing supply of donor corneas, even someday growing tissue on demand.
Though endothelial keratoplasty for Fuchs’ endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy has become increasingly successful, the basic prerequisite is availability of good quality donor corneas. New keratoplasty techniques under investigation that minimise the need for a donor cornea include Descemet stripping only (DSO) and Descemet membrane transplantation (DMT).
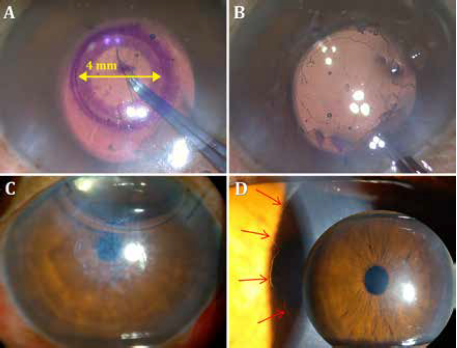
DSO does away with the transplant altogether, waiting for the patient’s endothelial cells to migrate into place. With DSO, the central 4 mm of Descemet’s membrane is removed to allow central migration of healthy peripheral cells for central oedema clearance over the next three to four months. DMT transplants a decellularized DM following the central stripping.
DSO and DMT are indicated only in FECD with mild central oedema or central guttae and sufficient peripheral endothelial cell reserve (> 1400 cells/mm2). Rough Descemet membrane edges and stromal scoring interfere with central endothelial migration and should be avoided. Rho-kinase (ROCK) inhibitors can augment cell migration.
Patients considered for these procedures need preoperative counselling regarding the slow clearance of oedema and the possible need for EK in the eventuality of non-clearance by four months after surgery. Long-term steroids and rejection are avoided in DMT since the decellularized Descemet membrane is non-immunogenic. Non-optical-grade corneas can be used in DMT. The reliance on high-quality donor cornea is removed both for DMT and DSO procedures.
ROCK inhibitors impede apoptosis and promote endothelial cell proliferation, adhesion, and migration. They play a role in many of the new treatments for endothelial disease and are thought to enhance the results of endothelial keratoplasty, DSO, and DMT. They are also used in cell therapy cultivation methods.
Corneal endothelial cell therapy
Corneal tissue engineering aims to overcome donor tissue shortages and eliminate immune rejection. The ideal cell therapy would be non-toxic, immunologically compatible, genetically stable, free of transmissible diseases, and have long-term, stable functionality and survival. Important steps in cell therapy are tissue removal, isolation, differentiation, and cultivation in vitro, followed by implantation into the patient.
The challenges of this therapy include difficulty in obtaining a monolayer of cells with flat hexagonal morphology, programmed cell cycle arrest, avoiding morphological deviations, fibroblastic contamination, premature cellular senescence, and the need for a biocompatible carrier.
Human corneal endothelial cells (CEnC) possess limited proliferative ability within the eye since the cells are arrested in the quiescent G1 stage of the cell cycle. However, they may be induced to grow in vitro. Cell lines are obtained either from corneal endothelial stem cells or direct expansion. Descemet’s membrane is peeled from a corneal graft and digested using enzymes. The remaining cells are then cultured in vitro. Challenges include the risk of allogenic graft rejection and the limited regenerative capacity of donor cells.
Autologous adult stem cells under research include skin-derived precursors (SKP), mesenchymal stem cells (MSCs), peripheral blood monocyte cells (PBMCs), induced pluripotent stem cells (iPSCs), and even fibroblasts. MSCs are readily available in adipose tissue, bone marrow, and skeletal muscle. Ethical issues are few, and the risk of tumorigenesis is low. Disadvantages of stem cell therapy include variable differentiation capacity and differences in morphology.
Kinoshita’s landmark research
Following decades of research, Professor Shigeru Kinoshita and colleagues reported success in culturing corneal endothelial cells and implanting them in patients. This opens the door to taking a donor cornea, stripping out corneal endothelial cells, and growing it in the lab, thereby showing real potential for treating multiple patients from one donor cornea.
In the only published trial of endothelial cell therapy in humans, Prof Kinoshita and colleagues report that 11 bullous keratopathy patients received intracameral injections of in-vitro expanded CEnCs treated with a ROCK inhibitor, followed by prone positioning for 3 hours to achieve attachment to recipient corneas.1 After 24 weeks, corneal oedema regressed and CEnC density exceeded 500 cells per square millimetre (range 947 to 2833) in all eyes. Vision improved by two lines or more in 9 of the 11 patients, and the results were stable for up to 24 months.
Caveats include the inability to rule out the possibility of unattached CEnCs seeding systemically via the trabecular meshwork with subsequent tumorigenesis, a potential safety issue.
The techniques developed by Prof Kinoshita have been licenced to two companies—Aurion Biotech in the US and ActualEyes in Japan—with clinical trials underway. Proponents of this approach believe it may soon be possible to treat 50 patients or more from a single donor cornea.
An intact DM is essential for cell injection therapy. It is, therefore, preferable in early disease. Cell delivery systems such as superparamagnetic embedding and iron endocytosed CEnCs, are under investigation to increase adhesion. One novel approach, EO2002 (Emmecell), uses a magnetic cell delivery nanoparticle platform and a magnetic patch over the closed eyelid for a few hours after intracameral injection of CEnCs.
Tissue engineering and beyond
Tissue-engineered endothelial keratoplasty (TE-EK) is another approach under development. In this procedure, cells are cultured and differentiated onto supports, which are then implanted into the patient’s eye using standard endothelial keratoplasty techniques. The ideal support should be easily implantable, biocompatible, and biodegradable. Support materials tried thus far include ultrathin human stromal lamellae, anterior lens capsule, amniotic membrane, DM, and various biomimetic scaffolds of natural (collagen, gelatin) or synthetic (polyvinyl alcohol, polyethylene glycol) materials.
TE-EK is preferable in advanced disease when the recipient DM is scarred and needs removal. Large guttae are toxic to injected cells, and DM removal is indicated, again making TE-EK preferable.
EndoArt (EyeYon Medical), a 50-micron artificial lamella positioned similarly to EK, deturgesces the cornea by acting as a fluid barrier, helping to avoid long-term steroids and rejection. There are also no concerns about damaging the graft or disease transmission.
The device is approved in the US and Europe in patients with corneal oedema following graft rejection or those at risk of graft rejection.
Gene therapy offers the promise of a long-term cure. The cornea is ideal for gene therapy because of easy access and immune privilege. Gene therapy’s previous use improved allograft survival by transducing the donor cornea before transplantation and inducing regression of host corneal neovascularization. It is also being tried in eye bank corneas to maintain endothelial cell density, thus allowing long-term corneal preservation and making donor corneas with low cell density viable for transplantation. Identified biomarkers may serve as potential therapeutic targets for treating FECD.
Gene therapy has been used in mouse models to treat early-onset FECD. Viral and non-viral vectors and techniques to introduce genes into the cornea are being researched, showing how gene editing and CRISPR technologies may offer breakthroughs in FECD.
For citation notes, see page 46.
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
The 5 Ws of Post-Presbyopic IOL Enhancement
Fine-tuning refractive outcomes to meet patient expectations.
AI Shows Promise for Meibography Grading
Study demonstrates accuracy in detecting abnormalities and subtle changes in meibomian glands.
Are There Differences Between Male and Female Eyes?
TOGA Session panel underlined the need for more studies on gender differences.
Simulating Laser Vision Correction Outcomes
Individualised planning models could reduce ectasia risk and improve outcomes.
Need to Know: Aberrations, Aberrometry, and Aberropia
Understanding the nomenclature and techniques.
When Is It Time to Remove a Phakic IOL?
Close monitoring of endothelial cell loss in phakic IOL patients and timely explantation may avoid surgical complications.
Delivering Uncompromising Cataract Care
Expert panel considers tips and tricks for cataracts and compromised corneas.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.