European Society of Cataract and Refractive Surgeons (ESCRS) Guideline for Cataract Surgery
Abstract
Executive Summary
This is an Executive Summary version of the Clinical Practice Guidelines to be evaluated by stakeholders such as ESCRS members, sister societies, manufacturers as well as any other health professionals interested. We have previously invited comments from stakeholders until 31 October 2024. If you have any queries regarding these guidelines please contact the ESCRS Head Office cataract.guidelines@office.escrs.org
Background: Cataract is a leading cause of visual impairment, and surgical intervention remains the exclusive effective approach for vision restoration. Currently, there are no non-surgical therapeutic modalities to address this ocular disease. Furthermore, the prevalence of cataracts increases with age, significantly augmenting its impact on global visual health. The patient pathway for cataract management includes diagnostic and therapeutic steps such as screening, patient selection, preoperative diagnostics, treatment strategies, surgery itself and postoperative care.
Objective: To establish evidence-based guidelines by the European Society of Cataract and Refractive Surgeons (ESCRS) to support patients, clinicians and other relevant stakeholders in decisions about cataract management.
Methods: The ESCRS formed a multidisciplinary guideline panel balanced to minimize potential bias from conflicts of interest. The panel prioritized clinical questions and outcomes according to their importance for clinicians and patients. The guideline-development process, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, including GRADE Evidence-to-Decision framework, and was supported by a team of methodologists.
Results: The panel agreed on recommendations concerning 31 questions for patient pathway for cataract managements.
Conclusions: Key recommendations of these guidelines include (according to level of evidence):
- An intracameral injection should be used (e.g. cefuroxime 1 mg in 0.1 ml.) at the end of the cataract surgery to lower the risk for postoperative endophthalmitis. (GRADE +++)
- Topical anesthesia appears to be the most used anesthesia technique during cataract surgery, if suitable for the patient. (GRADE ++/+++) For further reducing pain during the cataract surgery, an additional intracameral lidocaine injection can be considered. (GRADE ++/+++)
- Toric IOLs should be considered in eyes with a degree of corneal astigmatism of 1.0D or more, with strong evidence for corneal astigmatism above 2.0D, moderate evidence for corneal astigmatism above 1.5D, and may be beneficial above 1.0D. (GRADE ++)
- The selection of a specific target refraction highly depends on the selected IOL, expectations and preferences of the patient. The patient and ophthalmologist should take the shared decision for IOL target selection. (GRADE ++)
- The primary treatment options for CME after cataract surgery are topical NSAIDs or steroids. However, there is a lack of sufficient evidence to establish the optimal treatment approach for this condition. (GRADE ++)
- Both conventional cataract surgery (CCS) and femtosecond laser assisted cataract surgery (FLACS) can be used as they are both safe and effective procedures. (GRADE +/++). They give comparable visual acuity and refractive outcomes and overall intraoperative and postoperative complication rates. (GRADE +/++)
- A combination of NSAIDs and corticosteroid eye drops is more effective to use after routine cataract surgery to prevent inflammation and CME compared to monotherapy. (GRADE +/++)
- In diabetic patients without diabetic retinopathy, it is recommended to use a combination of corticosteroid and non-steroidal anti-inflammatory drug (NSAID) eye drops to prevent cystoid macular edema. (GRADE +/++) In patients with diabetic retinopathy, a supplementary depot of triamcinolone should be considered to reduce this risk. Intraocular pressure must be monitored postoperatively when using a triamcinolone depot. (GRADE +)
- ISBCS (Immediate Sequential Bilateral Cataract Surgery) is effective and safe, has a high degree of patient satisfaction and can be considered in patients without complication-inducing ocular comorbidities. (GRADE +)
- EDF IOLs or pseudophakic monovision can be recommended for patients who desire a good intermediate visual acuity, with significantly less dysphotopsia compared to patients who received multifocal IOLs. (GRADE +)
- In general, posterior segment OCT in cataract surgery should be used when there is a clinical indication, such as age-related macular degeneration, diabetic retinopathy, glaucoma, or when the visual acuity is worse than expected. (GRADE +)
- Patient selection for pseudophakic presbyopia correcting IOLs should be based on the presence of ocular comorbidities, the desire for spectacle independence, and realistic patient expectations. (GRADE +)
- In the case of implantation of a toric IOL the preoperative assessment should encompass not only general mandatory evaluations but also corneal topography and/or tomography. (GRADE +) Methods which include measurements of factors such as the additional posterior corneal astigmatism and effective lens position are preferred for toric IOL calculation. (GRADE +)
- Specific IOL formulae are recommended for eyes with certain conditions to ensure accurate outcomes. In extreme long and short eyes new-generation formulae are recommended. (GRADE +)
- Postoperative remote care after cataract surgery might replace short-term clinical examination to better allocate hospital resources and increase time and cost efficiency. Accuracy and validity of remote care and telemonitoring are still to be evaluated. (GRADE +)
FINAL VERSION Cataract Guidelines Extended document
Keywords: Cataract, ophthalmology, practice guidelines, GRADE
List of abbreviations
ACD: Anterior chamber depth
AGREE II: Appraisal of Guidelines for Research and Evaluation II
AMD: Age-related Macular Degeneration
ASCRS: American Society of Cataract and Refractive Surgery
AL: axial length
CCS: Conventional Cataract Surgery
CDG: Constituting Guideline Development Group
CME: Cystoid Macular Edema
D: Diopter
DED: Dry eye disease
DSBCS: Delayed Sequential Bilateral Cataract Surgery
EDF: Extended Depth of Focus
ERM: Epiretinal Membrane
ESCRS: European Society of Cataract and Refractive Surgeons
FLACS: Femtosecond laser assisted cataract surgery
GDG: Guideline Development Group
GRADE: Grading of Recommendations Assessment, Development and Evaluation
IFIS: Intraoperative Floppy Iris Syndrome
IOL: Intraocular lens
IOP: Intraocular pressure
ISBCS: Immediate Sequential Bilateral Cataract Surgery
NSAID: Non-steroidal anti-inflammatory drug
OCT: Optical Coherence Tomography
PCO: Posterior Capsular Opacification
PEX: Pseudoexfoliation syndrome
PICO: Population Intervention Comparators Outcome
PROMS: Patient Reported Outcome Measures
RK: Radial Keratotomy
TASS: Toxic Anterior Segment Syndrome
TK: Total Keratometry
UDVA: Uncorrected Distance Visual Acuity
VEGF: Vascular Endothelial Growth Factor
1. Definitions
1.1 Definitions of target refraction
Prior to cataract surgery, patients should be consulted regarding the desired target refraction. This guideline covers the following target refraction goals:
- Emmetropia: Emmetropia refers to the condition in which there are no refractive errors present. When the eyes are in an emmetropic state, objects located at infinity are sharply focused on the retina without any need for accommodation. In practice, a refraction ranging between +0.25 diopters (D) and -0.25D is defined as emmetropia. (Langenbucher, 2015)
- Mini-monovision: Mini-monovision refers to the condition where one eye (usually the dominant eye) is targeted for emmetropia while the other eye (usually the non-dominant eye) is targeted for slight myopia ranging between -0.25D and -0.75D in order to increase spectacle independence. (Cochener, 2018)
- Monovision: Monovision refers to the condition when one eye is targeted for distance vision while the other eye is targeted for near vision. The range of diopters for monovision correction may vary according to the specific needs of the patient and discretion of the surgeon. In practice, monovision ranges from -1.00D to -2.00D. (AAO PPP Cataract and Anterior Segment Panel and Hoskins Center for Quality Eye Care, 2021 [accessed 2.5.23])
1.2 Definitions of different types of astigmatism
- Grading
- Low astigmatism (0.25 to 1.5D)
- Moderate astigmatism (1.5 to 3.0D)
- High astigmatism (above 3.0)
- Myopic / hyperopic / mixed astigmatism. (Nunez et al., 2019)
- Regular astigmatism
- With-the-rule astigmatism: Steep axis of the cylinder is vertical or within 30 degrees of the 90 degrees of vertical meridian (60-120 degrees)
- Against-the-rule astigmatism: Steep axis of the cylinder is horizontal or within 30 degrees of the horizontal meridian (0-30 or 150-180 degrees)
- Oblique astigmatism: Steep axis of the cylinder is not within 30 degrees of the horizontal or vertical meridians (31-59 degrees and 121-149 degrees)
- Irregular astigmatism
- Where the two main axes of astigmatism are not symmetric and/ or do not lie 90 degrees apart (orthogonal)
- Irregular or pathological astigmatism treatment is beyond the scope of these guidelines (e.g., those caused by corneal dystrophies, trauma, degeneration, ocular surface disease, corneal ectactic diseases such as keratoconus, and prior corneal surgery)
1.3 Definitions of different IOLs
Classifying intraocular lens (IOL) technologies is not an easy task, primarily due to the various categories that can be integrated into a classification. Some of these categories represent different characteristics that may be inappropriately combined in an attempt to create a simplified taxonomy, which is not always feasible. Therefore, when defining an IOL, it is crucial to differentiate between various categories and avoid mixing them, to prevent confusion for the user.
1.3.1 Optical Technologies
Two main types of optical technologies, diffractive and refractive, have historically been used to classify IOLs, depending on the optical principles utilized for focusing light. (Rampat and Gatinel, 2021) Diffraction and refraction can be achieved through distinct optical structures or optical features. (Davison and Simpson, 2006, Teng S, 2013) Diffraction can be accomplished with small optical apertures and diffractive gratings, while refraction can be achieved by varying the asphericity and radius of an optical surface or through zones and sectors.(Rampat and Gatinel, 2021, Teng S, 2013) However, some designs may combine some of the previously described optical features or mechanisms, thus controversies are likely to occur forcing to assign an IOL to one optical classification. (Kanclerz et al., 2020, Rampat and Gatinel, 2021, SM., 2021)
According to the shape and number of foci, a taxonomy with five categories has been described in the literature: multifocal intraocular lenses (including bifocal, trifocal and panfocal), with the first trifocal lens introduced in 2010,(Sudhir et al., 2019) extended depth of focus lenses (EDF), which emerged in 2014,(Kanclerz et al., 2020, Kohnen and Suryakumar, 2020) monofocal IOLs with enhanced depth of focus (Mono-EDF) for which the first Conformité Européenne (CE) mark was granted in 2019,(Fernández et al., 2023, Mencucci et al., 2020, Rampat and Gatinel, 2021) and conventional monofocal aspherical and finally spherical IOLs.
1.3.2 Standard Terms and Definitions
According to the International Organization for Standardization (ISO 11979-7, 2024), there are four main categories of IOLs that are determined by optical design and/or clinical characteristics or performance.(ISO-11979-7:2024., 2024) Monofocal, Toric, Simultaneous Vision Range Lenses (SVL) and Accommodating IOLs. From these, SVL are those non accommodative lenses that provide simultaneous vision at multiple distances and can be subclassified in three types:
- Multifocal (MIOL): emphasize optical and functionally useful acuity levels at far, but when compared to monofocal control lenses, also have improved optical and clinical performances at intermediate (for trifocal IOLs) and near distance.
- Extended Depth of Focus (EDF): emphasize optical and functionally useful acuity levels at far but also from far through intermediate distance.
- Full Visual Range (FVR): emphasize optical and functionally useful acuity levels at far but also from far through intermediate and up to near distance.
Table 2 describes the end-points that should be accomplished for classifying a SVL according to the ISO 11979-7:2024 standard
Table 2. Summary of end-points for Standard ISO 11979-7:2024 classification for Multifocal (MIOL), Extended Depth of Focus (EDF) and Full Visual Range lenses (FVR).
|
|
RoF for 0.2 logMAR (Diopters, D) |
RoF for 0.3 logMAR (Diopters, D) |
ΔVA (logMAR) |
|
1. PARTIAL-RoF |
< 2.3 |
< 2.75 |
0 |
|
1.1. Narrow |
< 1.2 |
< 1.61 |
0 |
|
1.2. Enhance |
≥1.2 and <1.58 |
≥1.61 and <1.98 |
0 |
|
1.3. Extend |
≥1.58 and <2.3 |
≥1.98 and <2.75 |
0 |
|
2. FULL-RoF |
≥ 2.3 |
≥ 2.75 |
≥ 0 |
|
2.1 Continuous |
≥ 2.3 |
≥ 2.75 |
< 0.05 |
|
2.2 Smooth Transition |
≥ 2.3 |
≥ 2.75 |
≥0.05 and <0.14 |
|
2.3 Steep Transition |
≥ 2.3 |
≥ 2.75 |
≥ 0.14 |
|
RoF: Range of Field; ΔVA: Visual Acuity increase from intermediate to near |
|||
It is important to note, that ISO agrees with the ANSI standard for EDF IOLs in all the end-points described in Table 2, but ISO adds the achievement of a RoF absolute value at a visual acuity level of 0.2 logMAR of 1.5 D.(Z80.35-2018-A.) Conversely, ANSI describes that EDF IOLs should have a monotonous decrease of visual acuity which means that the visual acuity from far to near should have a continuous decrease, and in the it should have an inflexion point, this one should be ≤ 0.04 logMAR.(Z80.35-2018-A.)
1.3.3 Evidence-Based Functional Classification
A functional classification has been developed considering the end-points described in the standards,(Fernandez et al., 2024) especially those referring to the RoF measured through monofocal visual acuity defocus curves with the best distance correction. This classification has been qualified as evidence-based because the scientific method (cluster analysis) has been the pillar during the development.
The cluster analysis found that two metrics were enough to classify IOLs:
- The increase in VA (ΔVA) from intermediate to near in the event of a non-monotonic decrease in visual acuity from far to near and
- The RoF at 0 D of defocus and at 0.2 logMAR or 0.3 logMAR cut-offs of visual acuity.
Figure 2 shows that two main categories can be identified depending on the defocus curve RoF at the visual acuity level of 0.2 logMAR and the shape: 1. PARTIAL-RoF, 2. FULL-RoF. In 1. PARTIAL-RoF three subcategories can be described according to the achieved RoF: 1.1. PARTIAL-RoF Extend, 1.2. PARTIAL-RoF Enhance, and 1.3. PARTIAL-RoF Narrow. On the other hand, 2. FULL-RoF IOLs subcategories depend on how steep is the increase in visual acuity from intermediate to near: 2.1. FULL-RoF Continuous for an increase below 0.05 logMAR, 2.2. FULL-RoF Smooth for an increase between 0.05 and 0.15 logMAR, and 2.3. FULL-RoF Steep for an increase above 0.15 logMAR.
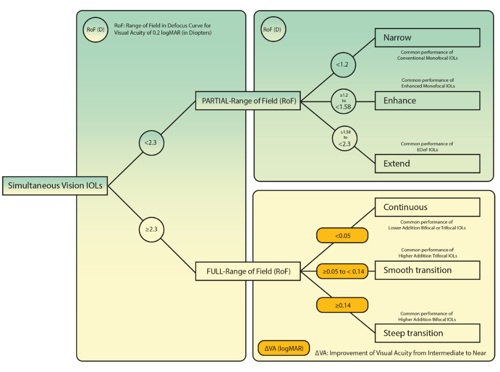
Figure 2: Diagram of functional classification depending on: 1. the range of the depth of field (RoF) achieved in the monocular defocus curve with best correction at distance at 0.2 logMAR visual acuity level, and 2. the improvement of visual acuity from intermediate to near ΔVA.
1.3.4 Conclusion
This IOL classification has been proposed by members of this ESCRS guideline development group. Historical terminologies used for classifying IOLs have been reviewed, and a functional classification has been introduced to enhance understanding, focusing on the visual acuity that patients achieve across the visual range. The importance of separating classification categories is emphasized, as different optical technologies could produce similar functional outcomes. However, there is a recognized relationship between optical terms and functional classification. Therefore, Table 3 summarizes the usual correspondences between optical, standard, and functional classifications, which may help surgeons understand the new concept.
Table 3. Summary of the usual correspondences between historical, standard, and functional terms.
|
Optical Technologies |
Standard Terms |
Functional Classification |
|
Monofocal |
Monofocal |
PARTIAL-RoF Narrow |
|
Enhanced Monofocal |
- |
PARTIAL-RoF Enhance |
|
EDF |
SVL: EDF |
PARTIAL-RoF Extend |
|
MIOL: Trifocal / Bifocal of low addition |
SVL: FVR |
FULL-RoF Continuous |
|
MIOL: Trifocal of high addition |
SVL: FVR |
FULL-RoF Smooth |
|
MIOL: Bifocal of high addition |
SVL: MIOL |
FULL-RoF Steep |
|
EDF: Extended Depth of Focus MIOL: Multifocal Intraocular Lenses SVL: Simultaneous Vision Range Lenses FVR: Full Visual Range RoF: Range of Field |
||
2. Introduction
Cataract is a significant cause of blindness, which is currently only reversible by surgery. As age advances, the prevalence of cataracts increases significantly. The prevalence of cataracts ranges from 3.9% among individuals aged 55-64 years to as high as 92.6% among those aged 80 years and older. It is projected that by 2025, the worldwide population of individuals affected by cataract blindness will increase to 40 million. (Fang et al., 2022) Cataract surgery is the most commonly performed surgical procedure worldwide, with 7 million cases each year in Europe, 3.7 million cases in the USA, and 20 million worldwide. (Rossi et al., 2021)
The purpose of the ESCRS Cataract Surgery Guidelines is to address the value of diagnostic and therapeutic steps for various stakeholders in the patient cataract care pathway. The guideline aims to apply to all healthcare workers (e.g., ophthalmologists, residents, general practitioners, nurses, optometrists, opticians, health care decision takers, patient societies, and health care insurance companies) and patients interested in cataract management. It provides explicit, evidence-based recommendations and insights that healthcare providers should follow to deliver high-quality care. The clinical recommendations are crucial for supporting clinical decision-making and promoting better care, transparency, and reduced unwanted practice variation. While they are not prescriptive regulations, healthcare providers may deviate from these recommendations in complex cases where the patient's circumstances differ significantly from the 'average patient.' The physician must exercise his/her judgment on the suitability of the care provided to a particular patient, considering all the circumstances presented by the patient. However, any deviation should be documented in the patient's record and be supported by clear reasoning.
3. Methodology
This guideline was developed according to the comprehensive quality criteria as described in the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument. (Dans and Dans, 2010) The guideline development group (GDG) consisted of 18 ophthalmologists, and the constituting guideline development group (CDG) of two PhD students and a supervising methodologist. All clinical members possessed expertise in diagnosing and treating cataracts, and they were geographically distributed to cater to diverse regions. Review questions were formulated according to the PICO framework. Outcome parameters were selected based on importance for decision-making in the clinical setting. Literature searches were performed, using KSR evidence, CDSR, Medline, Embase, and Central as resources to identify relevant systematic reviews and randomized controlled trials for each review question, in total 5744 articles were found. The two PhD-students performed selection of the articles supervised by the methodologist. Critical appraisal of available systematic reviews was performed based on the Risk of Bias assessment Tool ROBIS by reviewers at KSR Ltd.(Whiting et al., 2016) The quality of the relevant evidence was summarized using the GRADE approach. (Neumann et al., 2014) According to the GRADE approach, the evidence is classified as high (++++), moderate (+++), low (++) or very low (+). These classifications are accompanied by a specific formulation of the recommendations, using the wording ‘must’, ‘should’, ‘could’, ‘may’, ‘may not’, and ‘can be considered’. Considering high-level evidence, the term ‘must’ was used in the recommendations of this guideline. In the case of moderate evidence, ‘should’ or ‘could’ were used. For low-graded evidence, ‘could’ or ‘may’ are applicable, and lastly, when there was very low evidence implemented in the recommendations, ‘can be considered’ was used. (Whiting et al., 2016, Joanna Briggs Institute, 2020 [accessed 20.2.23], Neumann et al., 2014)
4. Summary of Recommendations
The structure of this guideline is according to the patient cataract care pathway, and includes following sections: screening and patient selection, preoperative assessment, perioperative procedure, postoperative care, and complications.
4.1 Screening and patient selection
4.1.1 What are the indications for cataract surgery? (Question 4.1)
A cataract is clinically diagnosed at the slit lamp examination by an ophthalmologist. The patient and ophthalmologist should take the shared decision for cataract surgery, and this should be well documented in the patient's medical records. (GRADE +)
Important aspects to be considered for the indication for cataract surgery are the presence and appearance of cataract, a patient’s visual acuity and function (visual acuity and quality of vision), the subjective disability of the patient and the expected benefits of the cataract surgery. (GRADE +).
Co-morbidities and the surgical risk profile should be considered and discussed with the patient prior to the surgery. Documentation of this process in the patient file is mandatory. It is recommended to use validated patient satisfaction questionnaires and Patient Reported Outcome Measures (PROMs) to evaluate the outcome of cataract surgery. (GRADE +)
4.1.2 Will the presence versus absence of (characteristic A) impact efficacy and safety outcomes in patients for whom cataract surgery is considered? (Question 4.2)
The presence of patient characteristics and comorbidities can have an impact not only on the outcome in vision but also on the risks of surgery and postoperative complications. These should be discussed with the patient so that they are fully informed, and expectations are realistic. (GRADE +)
Significant ocular comorbidities, such as diabetic retinopathy, glaucoma, maculopathies, and uveitis should be identified during the preoperative evaluation since its presence can affect the postoperative visual acuity and function. (GRADE +)
Additionally, certain risk factors can lead to a higher complication rate during cataract surgery including: pseudoexfoliation syndrome, Fuchs endothelial dystrophy, shallow anterior chambers, white and brunescent cataracts, small pupils, and eyes with an extreme axial length (<22mm or >26mm). In patients who previously underwent refractive surgery specific precautions should be taken to prevent a refractive surprise.
Regarding eyes with ‘active disease’ (such as uveitis, proliferative diabetic retinopathy, neovascular AMD, herpetic keratitis), these may be more susceptible to complications when performing cataract surgery.
It is recommended to perform the surgery when the disease is in a quiet phase. Customizing the postoperative follow-up based on the presence of ocular comorbidities is essential for ensuring comprehensive and effective care. Furthermore, it is crucial to provide patients with thorough information about potential risks to ensure realistic expectations. (GRADE +)
|
Comorbidity |
Recommendation |
|
Macular degeneration |
Cataract surgery in patients with macular degeneration improves visual function in all severity grades of AMD, at least in the short-term. (GRADE +) It is recommended to perform cataract surgery in a period of quiescence of neovascular AMD, but timing for cataract surgery must be individualized according to the patient’s needs (GRADE +) Maculopathies should be identified before cataract surgery. The long-term effects of AMD after cataract surgery are still unclear. (GRADE +) |
|
Glaucoma/ Ocular hypertension |
Intraocular pressure (IOP) should be monitored short-term after cataract surgery in glaucoma patients. (GRADE ++) |
|
Diabetic Retinopathy |
The presence of active diabetic retinopathy or diabetic macular edema increases the risks of intraoperative and postoperative complications, such as macular edema. (GRADE +) Cataract surgery can be considered when the underlying retinal disease is stabilised or when the presence of the cataract impedes with the evaluation and treatment of the retinal disease. (GRADE +) |
|
Dry eye disease (DED) |
Preoperative dry eye has an impact not only on preoperative examinations but also on postoperative outcomes. DED management should be optimised prior to surgery (see preoperative examinations) and the patients should be informed that DED symptoms often become worse after surgery (although often temporary). (GRADE +) |
|
Amblyopia |
Preoperative orthoptic examination may be necessary to assess binocular vision and detect possible amblyopia. (GRADE +) |
|
Corneal opacities |
Patients should be advised on the impact of the opacities on outcomes and the risk of additional medical or surgical management. (GRADE +) |
|
Macular pucker/ epiretinal membrane (ERM) |
Compared to eyes without ERM, higher rates of cystoid macular edema and a reduced postoperative gain in visual acuity can be noted. (GRADE +) |
|
Previous refractive surgery |
In cataract patients who previously underwent refractive surgery, special preoperative examinations such as corneal topography and tomography may be of added value. (GRADE +) The impact of previous surgery on refractive outcome prediction should be discussed as well as the need for further refractive correction. (GRADE +) |
|
Shallow anterior chamber (ACD) |
Patients with a shallow anterior chamber should be informed about the increased risk of peri- and postoperative complications such as iris prolapse and corneal endothelial cell loss. (GRADE +) |
|
Pseudoexfoliation syndrome (PEX) |
PEX is an important risk factor in phacoemulsification because of complications such as poor pupillary dilatation, zonular weakness inducing intra- or postoperative lens dislocation, vitreous loss, postoperative IOP spikes, capsular phimosis, prolonged inflammation, and postoperative corneal decompensation. Patients should be counselled accordingly. (GRADE +) |
|
White/ Brunescent cataract |
Surgical adaptions such as using trypan blue for capsular bag staining and decompression/ aspiration, or “milking” of the cortical material should be performed to reduce the risk of capsular tear. (GRADE +) |
|
Small Pupil/ Intraoperative floppy iris syndrome (IFIS) |
The use of pupil expansion strategies should be considered in cases with small pupils that cannot be dilated pharmacologically. (GRADE +). In cases of IFIS a combination of strategies including appropriate phacoemulsification fluidic parameters, pharmacological agents, longer corneal tunnels and dispersive viscoelastics should be considered. (GRADE +) |
|
Eyes with extreme axial length |
Long eyes can be defined as eyes with an axial length of over 26mm, while short eyes are generally defined as eyes with an axial length of under 22mm. |
|
Uveitis |
Patients with uveitis will have visual improvement after cataract surgery but are also at more risk for the development of macular edema and a recurrence of uveitis. (GRADE +) Active inflammation should be controlled prior to surgery, which means that the inflammation is sufficiently controlled, where possible. (GRADE +) |
|
History of herpes keratitis |
Antiviral prophylaxis with acyclovir or valacyclovir should be considered. (GRADE +) |
|
Vascular occlusions |
Patients with preoperative history of central retinal vein occlusions should be informed about the possible limitations on their visual outcomes after cataract surgery compared to other patients without retinal diseases. (GRADE +) |
4.1.3 What mental health factors must be considered when preparing for cataract surgery? (Question 4.3)
Cataract surgery has a beneficial effect on cognitive and mental health. Cataract extraction can be considered in patients at higher risk for cognitive decline and impaired vision due to cataracts. (GRADE +/++)
The timing of the surgery with regard to the course of the mental illness and the potential application of Immediate Sequential Bilateral Cataract Surgery (ISBCS) may be of benefit for these patients. (GRADE +/++)
4.1.4 What information about surgery, target refraction and complications should be given to the patient before cataract surgery? (Question 4.4)
The ophthalmic surgeon should ensure that the following information is verbally provided to the patient before obtaining informed consent and performing cataract surgery:
- The option of not undergoing surgery
- Purpose and nature of the cataract surgery
- Risk for (serious) complications during and after the surgery
- Patient-specific additional risks
- Surgery on one or both eyes
- Target refraction and expected vision improvement after surgery
- Treatment options: IOL types
- The financial implications of the surgical and IOL choices
- In cases of bilateral cataract surgery: delayed sequential or immediate bilateral surgery
- Type of anaesthesia
- What to do in emergencies
Targeted interventions improve patients’ satisfaction with cataract surgery and the accompanying postoperative care. (GRADE +)
In addition to verbal information, patients undergoing cataract surgery should be provided with written information and, if possible, audiovisual material. It is important to consider national informed consent guidelines and adapt the information provided to local best practices and legal frameworks. (GRADE +)
4.1.5 In patients needing cataract surgery, what are the effects of immediate bilateral surgery compared with delayed sequential surgery and what is the minimum time between cataract surgery on the first and second eye? (Question 4.5)
ISBCS (Immediate Sequential Bilateral Cataract Surgery) is effective and safe, has a high degree of patient satisfaction and can be considered in patients without complication-inducing ocular comorbidities. (GRADE +)
There are comparable clinical outcomes of DSBCS (Delayed Sequential Bilateral Cataract Surgery) and ISBCS. Therefore, either technique can be considered. (GRADE +/+++ (for endophthalmitis))
Bilateral cataract surgery on the same day allows rapid patient rehabilitation and helps avoid suboptimal visual function while waiting for second-eye surgery. However, there was no extra long-term benefit of self-assessed visual function compared with cataract surgery in one eye at a time. (GRADE +)
Specific relative contraindications must be considered if bilateral simultaneous cataract surgery is planned:
- The ISBCS should be reconsidered if there is an increased risk of peri- or postoperative complications.
- If complications occur during surgery of the first eye, these adverse events have to be resolved before proceeding to the second eye and delaying the second eye should be considered.
If bilateral simultaneous cataract surgery is planned, it should be considered and treated as two entirely separate procedures, according to the principal practice guideline for bilateral surgery. (RCO, 2020) The main statements of this guideline include: the instruments go through separate sterilization cycles with indicators; concomitant ocular or periocular disease should be controlled and managed before surgery; and, any issues with the first eye surgery must be resolved before proceeding with the second eye.
4.1.6 Do pseudophakic presbyopia correcting IOLs have a better postoperative outcome than monofocal IOLs or monofocals with monovision? (Question 4.6)
Detailed patient information must be given to choose the correct IOL type for patients undergoing cataract surgery with pseudophakic correcting presbyopia IOLs. (GRADE +)
Multifocal IOLs should be considered in patients who desire a high chance of spectacle independence for far, near and intermediate vision, as multifocal IOLs show better results than standard monofocal IOLs in uncorrected near and intermediate vision. (Low certainty evidence for bifocal vs. trifocal)
Thoughtful use of multifocal IOLs is recommended, as unwanted visual phenomena e.g. halos, glare and dysphotopsia are more common in multifocal IOLs than in monofocal IOLs. (GRADE +)
EDF IOLs or pseudophakic monovision can be recommended for patients who desire a good intermediate visual acuity, with significantly less dysphotopsia compared to patients who received multifocal IOLs. (GRADE +)
The implantation of EDF IOLs can be considered an effective method to treat some presbyopia with high rates of spectacle independence and minimal dysphotopsia side-effects and limited reading or near vision spectacle independent performance. (GRADE +)
|
Multifocal IOLs |
EDF |
Monovision |
|
Good near, intermediate and distance visual acuity |
Good intermediate and distance visual acuity |
Good intermediate and distance visual acuity |
|
Spectacle independence |
Spectacles for near vision needed in most cases |
Spectacles for near vision needed in most cases |
|
Higher probability of halos, glare, dysphotopsia, reduced contrast sensitivity |
Reduced probability of halos, glare, dysphotopsia |
Small probability of halos, glare, dysphotopsia |
|
Increased costs (depending on IOL/clinic) |
Increased costs (depending on IOL/ clinic) |
Mostly covered by insurance |
4.1.7 Do toric IOLs give a better postoperative outcome than non-toric IOLs in cataract surgery? From which magnitude of corneal astigmatism is a toric IOL indicated? (Question 4.7)
Current recommendations are based on studies performed using anterior keratometry:
In the case of regular corneal astigmatism, toric IOLs may be considered for implementation. (GRADE ++)
Toric IOLs should be considered in eyes with a degree of corneal astigmatism of 1.0D or more, with strong evidence for corneal astigmatism above 2.0D, moderate evidence for corneal astigmatism above 1.5D, and may be beneficial above 1.0D. (GRADE ++)
New insights rely on predictions of postoperative astigmatism, making it imperative to use these predictions as a basis for decision-making in cases with corneal astigmatism.
4.1.8 What type of anesthesia is indicated for the patient? (Question 4.8)
There are several accepted and safe anesthesia techniques available for patients who undergo cataract surgery. Topical anesthesia appears to be the most used anesthesia technique during cataract surgery, if suitable for the patient. (GRADE ++/+++)
For further reducing pain during the cataract surgery, an additional intracameral lidocaine injection can be considered. (GRADE ++/+++)
The choice for a specific type of anesthesia during cataract surgery should be made together with the surgeon and patient. (GRADE +)
Nevertheless, other techniques have also gained acceptance and have demonstrated safety during cataract surgery. The decision-making process regarding the choice of anesthesia should be a collaborative effort involving the ophthalmologic surgeon and the patient. The anesthesia technique may vary from surgeon to surgeon, influenced by their experience and individual preferences. Additionally, patient-specific considerations, such as medical history, anxiety levels, and comfort, are vital in determining the most suitable anesthesia approach.
Under certain conditions, preoperative fasting and intravenous sedation can be considered. When general anesthesia is needed, preoperative health tests and blood samples might be necessary.
4.2 Preoperative assessment
4.2.1 What kind of diagnostics and preoperative assessment of the patient should be done? In patients who will undergo cataract surgery, what are the effects of diagnostic A versus no diagnostic A or versus diagnostic B on efficacy and safety outcomes? (Question 5.1)
Prior to cataract surgery, a series of essential preoperative examinations must be conducted to ensure a comprehensive understanding of the patient's ocular health and overall medical condition.
In general, for preoperative assessment prior to cataract surgery the following diagnostic measures are recommended: refraction, visual acuity, slit lamp assessment, biometry and tonometry. (GRADE ++)
Prior to surgery, it is recommended to provide patients with detailed patient information, including: (GRADE ++)
- Surgery process overview
- Potential intra- and postoperative complications
- Target refraction
- Various IOL options
- Postoperative care instructions and medications
In the presence of refractive astigmatism additional measurements with tomography/topography are recommended. (GRADE +)
It is recommended to use cataract-specific checklists adapted to the clinic of practice, since checklist use is associated with improvement of patient safety by reducing surgical morbidity and mortality. (GRADE +)
In addition to diagnostic measurements, providing detailed patient information is crucial. This includes discussing target refraction, potential visual outcomes, and postoperative instructions with the patient. Equally essential is providing a detailed explanation of the surgical process and potential complications.
4.2.2 What kind of diagnostics and preoperative assessments of patients who previously underwent refractive surgery should be done? (Question 5.2)
It is recommended to perform corneal tomography or topography in patients who previously underwent corneal refractive surgery. (GRADE +)
In post-radial keratotomy (RK) eyes a corneal tomography/ topography should be performed for assessment of corneal astigmatism and corneal irregularities. (GRADE +)
A slightly myopic target refraction should be considered. (GRADE +) Patients should be informed about the possibility of a refractive surprise. (GRADE +)
It is recommended to choose a slightly myopic target refraction in anterior and posterior phakic IOLs. (GRADE +) An anterior segment OCT may be used for axial length and anterior chamber depth measurements in anterior phakic IOLs. (GRADE +) Endothelial cell count should be done in patients with anterior phakic IOLs prior to cataract surgery. (GRADE +)
4.2.3 What kind of diagnostics and preoperative assessments of patients who previously underwent refractive surgery should be done? (Question 5.2)
In general, posterior segment OCT in cataract surgery should be used when there is a clinical indication, such as age-related macular degeneration, diabetic retinopathy, glaucoma, or when the visual acuity is worse than expected. (GRADE +)
OCT is more effective in detecting optic nerve or macular pathologies than a regular fundus examination. (GRADE +)
Posterior segment OCT may be used in routine cataract cases and can be considered at least in the following situations: (GRADE +)
- In case of increased risk or medical history of macular abnormalities that could adversely affect the postoperative visual outcome, such as AMD, diabetic retinopathy
- Where the visual acuity is worse than expected and cannot be fully explained by the degree of cataract
- In case of considering presbyopia correction IOLs.
4.2.4 In which patients with an indication for cataract surgery is ultrasonography (A- or B-scan) indicated? (Question 5.4)
Ultrasound biometry (A- and/or B-scan) should be used when there is low visibility of the posterior segment, such as in mature and dense cataracts when optical biometry is not applicable or feasible. (GRADE +) An A-scan provides valuable information about the axial length, while B-scan offers detailed imaging of the posterior segment, enabling the detection of any underlying ocular conditions that may impact surgical planning and outcomes.
4.2.5 What are the indications for specific assessment examinations for patients with corneal comorbidities? (Question 5.5)
Pre-existing corneal comorbidities, including dry eye disease, Fuchs’ endothelial dystrophy and corneal scars and opacities are crucial to identify before performing cataract surgery since this may influence the outcomes.
Consider treatment of the dry eye disease before performing cataract surgery. Patients with pre-existing dry eye disease should be recognized and diagnosed before cataract surgery by testing the tear breakup time, corneal fluorescein staining or Schirmer test. (GRADE +)
The severity of Fuchs’ endothelial dystrophy should be evaluated for cataract surgery decision-making, based on the clinical presentation and the visual symptoms. (GRADE +)
Identifying corneal scars or opacities before cataract surgery is important for estimating potential vision gain. If a patient is not a good candidate for corneal transplantation, phacoemulsification can still be safely performed. In addition, cataract surgery can serve as an interim measure while the patient waits for penetrating keratoplasty. (GRADE +)
4.2.6 What are the indications for specific assessment examinations for patients with keratoconus? (Question 5.6)
In keratoconus patients, stabilizing procedures before cataract surgery should be considered if the patient is at risk of progression. (GRADE +)
When evaluating astigmatism in this patient population, the anterior, posterior and total corneal astigmatism should be assessed to perform the most accurate IOL calculations. (GRADE +)
4.2.7 What preoperative assessment is necessary for presbyopia correcting IOLs? (Question 5.7)
Patient selection for pseudophakic presbyopia correcting IOLs should be based on the presence of ocular comorbidities, the desire for spectacle independence, and realistic patient expectations. (GRADE +)
For the preoperative assessment before implantation of a presbyopia correcting IOL (including monofocal+, EDF, multifocal IOLs), besides the general mandatory preoperative assessment (see chapter 5.1), additional assessments can be considered including evaluation of dry eye symptoms, stereopsis assessment, corneal topography/tomography, posterior segment OCT, and pupillometry. (GRADE +)
4.2.8 What preoperative assessment is necessary for toric IOLs? (Question 5.8)
In the case of implantation of a toric IOL the preoperative assessment should encompass not only general mandatory evaluations but also corneal topography and/or tomography. (GRADE +) Methods which include measurements of factors such as the additional posterior corneal astigmatism and effective lens position are preferred for toric IOL calculation. (GRADE +)
4.3 IOL power calculation
4.3.1 Which formula(e) for calculating lens power should be considered? (Question 6.1)
There is a tendency towards improved outcomes with newer-generation formulae as they show less trend error, meaning that they appear more consistent along the range of axial lengths. Traditional formulae can still be considered an acceptable option where newer formulae are not available. (GRADE +)
4.3.2 Which formula(e) for calculating lens power in specific conditions should be considered? (Question 6.2)
Specific IOL formulae are recommended for eyes with certain conditions to ensure accurate outcomes. In extreme long and short eyes new-generation formulae are recommended. (GRADE +)
In eyes with keratoconus all formulae tend to result in a hyperopic surprise. It is recommended to avoid traditional formulae other than SRK/T and to use keratoconus-specific formulae for more accurate outcomes. It is suggested that the Barrett True-K and Kane formulae for keratoconus have more accurate results, especially in more advanced stages of keratoconus. (GRADE +)
In patients with steep corneas (>46D) or very flat corneas (<42D), the Barrett Universal II (Total Keratometry (TK)) and EVO (TK) formulae may be considered. (GRADE +)
The Haigis formula should be considered for patients with an ACD >3.5mm, while the Hoffer Q formula is suggested for a shallow anterior chamber (ACD <2.5mm). (GRADE +)
MICS (micro-incision cataract surgery) followed by the implantation of toric IOLs can be considered a safe and effective procedure for keratoconus patients regarding keratometric stability, visual and refractive results. (GRADE +)
4.3.3 Which formula(e) for calculating the intraocular lens in patients who have undergone refractive surgery is/are preferred? (Question 6.3)
When performing IOL calculations in patients who have undergone refractive surgery, designated formulas/methods should be used such as the American Society of Cataract and Refractive Surgery (ASCRS) post-refractive calculator. The ESCRS calculator will come out in September 2024. (GRADE +)
4.3.4 Which target refraction is preferred in patients who will undergo cataract surgery? (Question 6.4)
The selection of a specific target refraction highly depends on the selected IOL, expectations and preferences of the patient. The patient and ophthalmologist should take the shared decision for IOL target selection. (GRADE ++)
4.4 Perioperative procedure
4.4.1 What are the differences between femtosecond assisted laser cataract surgery (FLACS) and conventional phacoemulsification cataract surgery? (Question 7.1)
Both conventional cataract surgery (CCS) and femtosecond laser assisted cataract surgery (FLACS) can be used as:
- They are both safe and effective procedures. (GRADE +/++)
- Visual acuity and refractive outcomes are comparable. (GRADE +/++)
- Overall intraoperative and postoperative complication rates are low and appear similar for both conventional phacoemulsification and femtosecond laser assisted cataract surgery (GRADE +/++)
FLACS may be considered in patients with dense cataract or low endothelial cell count as it is a more effective method for reducing endothelial cell loss and postoperative central corneal thickening. Nonetheless, at 6 months postoperatively no significant differences were found between conventional cataract surgery and FLACS regarding endothelial cell loss. (GRADE +/++)
4.4.2 What is the role of femtosecond laser in astigmatism control during a cataract surgery? (Question 7.2)
Femtosecond-laser assisted (FLACS) as well as manual corneal incisions (eg. opposite clear corneal incisions, limbal relaxing incisions and astigmatic keratotomies) are safe and effective options for astigmatism control during cataract surgery. (GRADE +)
FLACS incisions for the main surgical incision are less effective than relaxing incisions in terms of effectivity and variability and should therefore only be considered in selected patients. (GRADE +)
Femtosecond laser can be used to perform corneal incisions specifically designed to correct corneal astigmatism (eg. intrastromal and penetrating femtosecond laser astigmatism keratotomies). These are more precisely performed than when done by hand. (GRADE +)
4.4.3 What are the differences between different marking techniques for patients receiving toric IOLs? (Question 7.3)
Image-guided marking may result in less axis misalignment, a smaller difference vector and less postoperative astigmatism than manual marking, but there are no clinically significant differences in visual and refractive outcomes between the two techniques. (GRADE +)
4.4.4 What prophylaxis should be administered during cataract surgery to minimize the risk of postoperative endophthalmitis? (Question 7.4)
Intracameral antibiotic therapy should be used because it is effective and safe for preventing endophthalmitis after cataract surgery. The use of intracameral antibiotics significantly reduces the risk of endophthalmitis. (GRADE +)
An intracameral injection should be used (e.g. cefuroxime 1 mg in 0.1 ml.) at the end of the cataract surgery to lower the risk for postoperative endophthalmitis. (GRADE +++)
Adequate antisepsis can be achieved by applying povidone-iodine 5-10% drops 3 minutes before commencing cataract surgery or by continuously applying 0.25% povidone-iodine drops to wash the ocular surface every 20-30 seconds during the procedure. In cases of povidone-iodine allergy, chlorhexidine (0.02%) can be used as an alternative. (GRADE +)
4.4.5 What prophylaxis should be used in cataract surgery to minimize the risk of post-operative inflammation? (Question 7.5)
- What is the most effective treatment to reduce post-operative inflammation after cataract surgery and reduce the risk of cystoid macular edema?
- Is perioperative inflammatory prophylaxis (dropless cataract surgery) equally effective as a postoperative anti-inflammatory eyedrop regimen?
A combination of NSAIDs and corticosteroid eye drops is more effective to use after routine cataract surgery to prevent inflammation and CME compared to monotherapy. (GRADE +/++)
It is currently unclear whether dropless inflammatory prophylaxis is as safe and effective as topical inflammatory prophylaxis to prevent CME and inflammation after cataract surgery. (GRADE +/++)
4.4.6 What are the optimal intra- and postoperative medication for patients with other ocular pathologies who undergo cataract surgery? (Question 7.6)
Specific postoperative treatments should be considered for patients with certain ocular comorbidities following cataract surgery.
In diabetic patients without diabetic retinopathy, it is recommended to use a combination of corticosteroid and non-steroidal anti-inflammatory drug (NSAID) eye drops to prevent cystoid macular edema. (GRADE +/++)
In patients with diabetic retinopathy, a supplementary depot of triamcinolone should be considered to reduce this risk. Intraocular pressure must be monitored postoperatively when using a triamcinolone depot. (GRADE +)
Literature reports discrepancies whether anti-vascular endothelial growth factor (VEGF) intravitreal intervention has no effect in preventing the occurrence of CME after cataract surgery in patients with diabetes. (GRADE +/++)
In patients with retinal diseases, topical NSAIDs should be used, and only in selected cases intravitreal anti-VEGF injections could be considered. (GRADE +)
In patients with uveitis, an increased frequency and prolonged treatment with steroids is suggested. Oral steroids should be applied only in specific cases. (GRADE +)
Glaucoma patients should receive carbonic anhydrase inhibitors postoperatively to minimize the potential increase in intraocular pressure (IOP) after surgery. A follow-up visit within one day after surgery is essential to monitor and control IOP. (GRADE +)
Oral acetazolamide administration postoperatively can be considered to reduce IOP elevation after cataract surgery. (GRADE +)
Patients with dry eye disease should use artificial tears both before and after surgery to manage symptoms and optimize ocular surface health. (GRADE +/++)
4.5 Postoperative care
4.5.1 What precautions does the patient have to consider after the surgery? When should the next follow-up visit take place? (Question 8.1)
The following precautions have to be considered after surgery: the patient should take the eye drops as instructed and seek help if vision decreased after prior vision increase, sudden appearance of black dots, flashing lights, increased pain or redness of the operated eye. Patients should not rub the eye, avoid getting water in the eye for at least one week, avoid activities that could strain the eyes for the first days after surgery, and cannot drive the car after surgery and have to wait until legal clearance. (GRADE +)
New glasses can be prescribed after 4-6 weeks. Uncomplicated cases can defer follow-up visits by up to two weeks without safety reduction. (GRADE +)
4.5.2 What is the preferred postoperative medication that should be administered to treat inflammation and CME after cataract surgery? (Question 8.2)
The incidence of clinically significant cystoid macular edema (CME) following cataract surgery has been reported to be as high as 2%. In many cases, the CME is a self-limiting condition that resolves spontaneously without any visual impairment.(Kessel et al., 2014) Nevertheless, there are instances where CME might persist or may lead to deterioration of the visual function which necessitates treatment. The primary treatment options for CME after cataract surgery are topical NSAIDs or steroids. However, there is a lack of sufficient evidence to establish the optimal treatment approach for this condition. This highlights the importance of conducting future research to further explore and clarify the most effective strategies for managing CME following cataract surgery. (GRADE ++)
No definitive conclusions can be drawn regarding the clinical effectiveness of injectable medications (including intravitreal injection of anti-VEGF, sub-tenon steroid injections and intravitreal steroid implants) for the treatment of CME. (GRADE +)
4.5.3 When is remote care after cataract surgery indicated for patients? (Question 8.3)
Postoperative remote care after cataract surgery might replace short-term clinical examination to better allocate hospital resources and increase time and cost efficiency. Accuracy and validity of remote care and telemonitoring are still to be evaluated. (GRADE +)
Screening has to be performed prior to allocating patients to a certain group that will receive remote care. Patients at an increased risk of complications or patients with comorbidities which may adversely affect their postoperative outcome should be prioritized for traditional postoperative hospital care. (GRADE +)
4.6 Complications
4.6.1 What kind of (serious) complications can occur during and after cataract surgery? (Question 9.1 and 9.2)
In general, cataract surgery is a procedure with a high safety profile and low incidence of complications. Complications can occur during or after the cataract surgery procedure, in the early or late postoperative period. The most important (serious) adverse events during cataract surgery include, posterior capsule rupture, dropped nucleus, zonular dialysis, iris or IOL damage, and suprachoroidal hemorrhage.
Important (serious) adverse events after cataract surgery include intraocular inflammations (including CME), endophthalmitis, toxic anterior segment syndrome (TASS), retinal detachments, pseudophakic bullous keratopathy, IOL related complications (including IOL dislocation, luxation, malposition, damage, opacification, calcification, remaining postoperative refractive errors, presence of photic phenomena), posterior capsular opacification, capsular contraction syndrome, and corneal edema.
4.6.2 What kind of adverse events can occur during cataract surgery? (Question 9.3 and 9.4)
Important adverse events that might occur during cataract surgery might include anterior capsule tear, iris damage and zonular dialysis without vitreous loss.
Adverse events that might occur after cataract surgery might include posterior capsular opacification (PCO), capsular contraction syndrome, elevated intraocular pressure, inflammation, corneal edema, binocular imbalance and double vision, dry eye symptoms as well as refractive surprise.
4.7 Cost-effectiveness
4.7.1 What is the cost-effectiveness of specific cataract surgery-related decisions? (Question 10)
A cost-effectiveness overview is provided in chapter 10, including the following topics: endophthalmitis prevention, prevention of inflammation/CME after cataract surgery, toric IOLs, Immediate sequential bilateral cataract surgery (ISBCS), and femtosecond laser assisted surgery.
5. Limitations and implementation considerations
The aim of this guideline is to provide comprehensive and evidence-based recommendations for managing cataract surgery. However, it is important to acknowledge certain limitations. Primarily, there are persistent evidence gaps in specific areas, which may necessitate careful interpretation of certain recommendations. The ESCRS has supported various clinical studies, including the Endophthalmitis Study and PREMED Study, but further research projects are needed to address the knowledge gaps identified in this guideline. Moreover, it is crucial to recognize that the guideline primarily focuses on routine cataract patients. Cases involving severe comorbidities may require individualized decision-making regarding outcomes or the surgery itself. Additionally, the implementation of these recommendations may face barriers due to the variability in clinical practices and workflows. It is important to acknowledge these potential barriers, as they may require tailored approaches in certain instances. Despite these limitations, this guideline serves as a valuable resource for all stakeholders involved in cataract care, providing evidence-informed guidance for cataract management.
6. Conclusion
This guideline presents valuable insights and practical recommendations for healthcare professionals engaged in cataract care, achieved through a rigorous review of evidence and expert consensus. By adhering to these evidence-based guidelines, clinicians can elevate the quality of care they deliver to patients, thereby enhancing outcomes and overall health. We urge stakeholders to integrate this guideline into their clinical decision-making processes, recognizing its capacity to instigate positive changes in healthcare practice and patient outcomes. It is important to note that this document is dynamic and can be adapted as new evidence emerges. The ESCRS has played a crucial role in optimizing future cataract care by providing a transparent, evidence-based guideline, and supporting clinical studies in the field.
7. Plans for updating these guidelines
The ESCRS aims to update its guidelines periodically, every 5 years. This process will involve conducting update searches and assessing any relevant research in relation to the current recommendations and considerations. The project team will evaluate whether modifications or revisions to the recommendations are warranted in light of emerging evidence or evolving best practices.
8. Conflicts of interest
Conflicts of interest can be found in the document linked below.
9. Members of the working group and authors
Chairs of the working group
- Prof. Oliver Findl
- Prof. Rudy Nuijts
First authors
- Dr. Joukje Wanten
- Dr. Victoria Till
Members of the cataract guidelines group
- Dr. Filomena Ribeiro
- Prof. Jorge Alio
- Prof. Anders Behndig
- Prof. Gerd Auffarth
- Dr. Nic Reus
- Prof. Sorcha Ni Dhubhghaill
- Dr. Mayank Nanavaty
- Dr. Catarina Pedrosa Priv. Doz.
- Dr. Nino Hirnschall
- Dr. Peter Hoffmann
- Dr. Adí Abulafia
- Prof. Dominique Monet
- Dr. Alex Day
- Dr. Roberto Belluci
Systematic reviewers
- KSR team (Jos Kleijnen and team)
References
AAO PPP Cataract and Anterior Segment Panel & Hoskins Center for Quality Eye Care 2021 [accessed 2.5.23]. Cataract in the adult eye PPP 2021 [Internet].
Cochener, B. 2018. Influence of the level of monovision on visual outcome with an extended range of vision intraocular lens. Clin Ophthalmol, 12, 2305-2312.
Dans, A. L. & Dans, L. F. 2010. Appraising a tool for guideline appraisal (the AGREE II instrument). Journal of Clinical Epidemiology, 63, 1281-1282.
Davison, J. A. & Simpson, M. J. 2006. History and development of the apodized diffractive intraocular lens. J Cataract Refract Surg, 32, 849-58.
Fang, R., Yu, Y. F., Li, E. J., et al. 2022. Global, regional, national burden and gender disparity of cataract: findings from the global burden of disease study 2019. BMC Public Health, 22, 2068.
Fernandez, J., Ribeiro, F., Rocha-de-Lossada, C. & Rodriguez-Vallejo, M. 2024. Functional Classification of Intraocular Lenses Based on Defocus Curves: A Scoping Review and Cluster Analysis. J Refract Surg, 40, e108-e116.
Fernández, J., Rocha-de-Lossada, C., Zamorano-Martín, F., et al. 2023. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol, 23, 101.
ISO-11979-7:2024. 2024. Part 7: Clinical investigations of intraocular lenses for the correction of aphakia.
Joanna Briggs Institute 2020 [accessed 20.2.23]. Checklist for randomized controlled trials: critical appraisal tools for use in JBI systematic reviews [Internet]. Adelaide: Joanna Briggs Institute.
Kanclerz, P., Toto, F., Grzybowski, A. & Alio, J. L. 2020. Extended Depth-of-Field Intraocular Lenses: An Update. Asia Pac J Ophthalmol (Phila), 9, 194-202.
Kessel, L., Tendal, B., Jorgensen, K. J., et al. 2014. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology, 121, 1915-24.
Kohnen, T. & Suryakumar, R. 2020. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg, 46, 298-304.
Langenbucher, A. 2015. Emmetropia, definition. In: Schmidt-Erfurth, U. & Kohnen, T. (eds.) Encyclopedia of Ophthalmology. Berlin, Heidelberg: Springer.
Mencucci, R., Cennamo, M., Venturi, D., et al. 2020. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg, 46, 378-387.
Neumann, I., Pantoja, T., Peñaloza, B., et al. 2014. El sistema GRADE: un cambio en la forma de evaluar la calidad de la evidencia y la fuerza de recomendaciones. Revista médica de Chile, 142, 630-635.
Nunez, M. X., Henriquez, M. A., Escaf, L. J., et al. 2019. Consensus on the management of astigmatism in cataract surgery. Clinical Ophthalmology, 13, 311-324.
Rampat, R. & Gatinel, D. 2021. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology, 128, e164-e185.
RCO, R. C. o. O. 2020. Immediate Sequential Bilateral Cataract Surgery (ISBCS) during COVID recovery: RCOphth/UKISCRS rapid advice document.
Rossi, T., Romano, M. R., Iannetta, D., et al. 2021. Cataract surgery practice patterns worldwide: a survey. BMJ Open Ophthalmol, 6, e000464.
SM., D. 2021. A discussion on modern IOL terminology. [Online]. Available: https://crstodayeurope.com/articles/feb-2021/lets-speak-lenspeak/ [Accessed February 23 2023].
Sudhir, R. R., Dey, A., Bhattacharrya, S. & Bahulayan, A. 2019. AcrySof IQ PanOptix Intraocular Lens Versus Extended Depth of Focus Intraocular Lens and Trifocal Intraocular Lens: A Clinical Overview. Asia Pac J Ophthalmol (Phila), 8, 335-349.
Teng S, L. G., Zhang C, Liu D. 2013. The diffraction by a small aperture. Opt - Int J Light Electron Opt., 124, 2507-2510.
Whiting, P., Savović, J., Higgins, J. P. T., et al. 2016. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology, 69, 225-234.
Z80.35-2018-A. Extended depth of focus intraocular lenses for presbyopia.
Disclaimer
These Clinical Practice Guidelines (CPGs) are provided by the European Society for Cataract and Refractive Surgeons (ESCRS). They represent current best practice in Europe, offering evidence-based recommendations. The CPGs are intended for use by healthcare professionals only to support clinical decision-making and promote best practice in clinical settings and as an educational tool.
These CPGs should not be taken as providing individualised medical advice, diagnosis or treatment. Healthcare professionals must make their own treatment decisions on a case-by-case basis, using their clinical judgement, knowledge and expertise and in consultation with the patient.
Specifically, these CPGs are not to be construed as the provision by ESCRS, the authors or the contributors of professionally qualified medical opinion or advice. Accordingly, none of the ESCRS, the authors or the contributors shall be liable for any direct, indirect, consequential or special loss or damage arising from or in connection with the use or misuse of these CPGs.
Any mention in these CPGs of any drug or commercial product is for informational purposes only and does not constitute an endorsement or recommendation.
These CPGs are protected by copyright. Reproduction or distribution of these CPGs for commercial purposes is strictly prohibited without prior written permission from ESCRS.
© ESCRS 2023. All rights reserved.